Transarterial chemoembolization (TACE) has been standard treatment for intermediate-stage hepatocellular carcinoma (HCC). However, all intermediate-stage HCC patients did not benefit from TACE treatment because intermediate-stage HCC encompasses a wide variety of HCCs. Owing to remarkable progress in systemic therapy, including molecular-targeted therapy for advanced-stage HCC, the standard treatment of HCC has recently shifted to systemic therapy.
- hepatocellular carcinoma
- intermediate-stage hepatocellular carcinoma
- molecular-targeted therapy
- tyrosine kinase inhibitor
- immune checkpoint inhibitor
- atezolizumab plus bevacizumab
- durvalumab plus tremelimumab
1. Introduction
2. The Definition and Standard Treatment of Intermediate-Stage HCC
In the Barcelona clinic liver cancer (BCLC) staging system, HCC is classified by tumor conditions (tumor number, maximum tumor size, the presence of vascular invasion, and the presence of extrahepatic lesions), liver reserve (Child–Pugh classification), and the Eastern Cooperative Oncology Group performance status (ECOG PS), and is divided into five stages: very early, early, intermediate, advanced, and terminal [10][11][10,11]. Intermediate-stage HCC was defined as the presence of four or more lesions, with liver functional preservation (Child–Pugh grade A or B), the absence of cancer-related symptoms (PS 0), and the absence of vascular invasion or extrahepatic spread beyond the BCLC-A criteria.2.1. Effectiveness of TACE in Intermediate-Stage HCC Treatment
In 2000, two randomized controlled trials (RCTs) comparing TACE and control groups were published. Lo et al. compared 40 randomized TACE patients (pts) with 39 control pts. Theis study reported 1-, 2-, and 3-year survival rates of 57%, 31%, and 26% in the TACE group and 32%, 11%, and 3% in the control group (relative risk 0.50, 95% confidence interval [CI]: 0.31–0.81, p = 0.005) [12]. Llovet et al. randomized 112 pts into three groups: 37 for transarterial embolization (TAE), 40 for TACE, and 35 for symptomatic therapy. The TACE group had a significantly better prognosis (p = 0.009) [13].2.2. Subclassification of Intermediate-Stage HCC
Intermediate-stage HCC involves a wide variety of HCCs with different pathologies, various tumor factors, and a reduction in hepatic functional reserve. Thus, Bolondi et al. proposed that intermediate-stage HCC should be divided into four substages (B1–B4) based on the following aspects: whether it is within or outside the up-to-7 criteria, which assess whether the sum of the number of tumors and the main tumor diameter exceeded 7; the Child–Pugh score; and the ECOG PS score [14][19]. The B1 stage was defined as an ECOG PS score of 0 with a Child–Pugh score of 7 points or less and within the up-to-7 criteria; TACE is the first-line treatment, and liver transplantation and combination therapy TACE with ablation are recommended as the alternative treatments [14][19]. Since then, several subclassifications have been proposed to refine this substage [15][16][17][20,21,22]. As the prognostic factors were four tumors and a tumor diameter of 7 cm, Yamakado et al. proposed the 4-of-7-cm criteria for the B1 stage (Child–Pugh score of 5–6 points and within 4-of-7-cm criteria), the B2 stage (Child–Pugh score of 7–8 points and 4-of-7-cm within criteria or Child–Pugh score of 5–8 points and 4-of-7-cm outside criteria), and the B3 stage (Child–Pugh score 9 points). With respect to the treatment, TACE should be performed in patients in B2 stage, while liver resection and local therapy are recommended in patients in B1 stage in addition to TACE. Meanwhile, patients in B3 stage are less likely to benefit from TACE [15][20].2.3. Switching from TACE to Drug Therapy as Treatment for Intermediate-Stage HCC
Sorafenib, a tyrosine kinase inhibitor (TKI), has emerged as a drug therapy for HCC, and repeated TACE may lead to gradual deterioration of the liver function, which may cause Child–Pugh class A patients to miss the opportunity to receive sorafenib. Therefore, concepts of TACE refractory and TACE inappropriate have been proposed [18][24]. Inappropriate TACE is a state in which the treated blood vessel is damaged and the catheter itself cannot be inserted, thus hindering the performance of TACE. It is also characterized by the deterioration of liver reserve function to Child–Pugh class C after repeated treatment and major portal vein invasion, making TACE technically impossible owing to the presence of a huge A-P shunt. TACE refractory is defined as the presence of intrahepatic lesions that are difficult to control even if TACE is performed twice, despite changing the drug and reconsidering other blood vessels for treatment. Additionally, the levels of tumor marker either do not decrease or they persistently increase, which is indicative of vascular invasion or distant metastasis [18][24].3. Advances in Cancer Immunotherapy
In HCC, the immunotherapy IMbrave150 (combination therapy with Atez and Bev) has shown positive results [8]. Subsequently, studies investigating other cancer immunotherapies, including COSMIC-312 [19][28], Checkmate 459 [20][29], KEYNOTE-240 [21][30], and the HIMALAYA trial, have been conducted [9]. The HIMALAYA trial compared the efficacy of combination therapy of Dur plus Tre with that of sorafenib [9]. The combination of Dur and Tre showed positive results compared with that of sorafenib treatment in this phase 3 trial [9]. These two regimens (Atez/Bev and Dur/Tre) have shown promising results and have been recommended as first-line treatments in the latest BCLC staging system [22][31]. However, post-progression TKIs have not yet been developed. The Imbrave150 trial demonstrated that treatment with a combination of monoclonal antibody targeting VEGF (Bev) and anti-PD-L1 inhibitor (Atez) improved the patients’ survival compared with sorafenib treatment alone in previously untreated patients [8]. The HR for death in patients treated with Atez/Bev was 0.58 (95% CI: 0.42–0.79). The OS and progression-free survival (PFS) were well stratified, with Atez/Bev improving both outcomes and quality of life compared with sorafenib. The HIMALAYA trial [9] revealed a significantly prolonged OS in the combination treatment group compared with that in the sorafenib group. Patients administered with Dur/Tre, the first approved combination therapy comprising anti-PD-L1 and anti-CTLA-4 antibodies for advanced-stage HCC, exhibited an HR of 0.76 (95% CI: 0.61–0.96). The COSMIC-312 trial compared the efficacy and safety of cabozantinib plus atezolizumab (Cab/Atez) with that of sorafenib as first-line therapy in patients with advanced-stage HCC [21][30]. The COSMIC-312 trial did not report an improvement in OS among patients receiving Cab/Atez. Furthermore, Atez/Bev treatment may be preferred in patients with WNT/β-catenin mutations and non-viral infections. Anti-VEGF therapies, including Atez/Bev and TKI regimens, improve the tumor microenvironment and produce moderate responses. By contrast, Dur/Tre may be less effective in patients with WNT/β-catenin mutations and non-viral-associated HCC. Although increasing evidence has shown that systemic therapy is effective and safe for patients with advanced-stage HCC, the specific regimens that are suitable for first-line treatment remain unknown.4. Combination of TACE and Molecular-Targeted Drugs
Systemic therapy using a highly responsive agent such as lenvatinib (LEN) in combination with subsequent selective TACE for residual tumors enhances the curative effect of TACE, preserves liver function, suppresses hypoxia-induced cytokines, and ultimately improves the patients’ survival. Therefore, the Asian Pacific Experts on Primary Liver Cancer (APPLE) [23][33] and the Japanese Society of Hepatology (JSH) [24][34] consensus statements recommend LEN as the first-line treatment for patients with intermediate-stage HCC who are not suitable for TACE. Recently, clinical practice guidelines from the E-updated European Society for Medical Oncology recommended upfront systemic therapy for patients who are ineligible for TACE [25][35].
The combined effect of TACE and molecular-targeted drugs can be explained as follows: the molecular-targeted drug acts on residual tumors caused by TACE, exerting anti-angiogenic and anti-tumor growth effects to prevent the exacerbation of residual tumors, avoid the development of new intrahepatic lesions, and suppress vascular invasion and distant metastasis. Molecular-targeted drugs with an anti-VEGF effect act on tumor blood vessels to improve the vascular permeability; reduce the tumor interstitial pressure; improve the drug delivery; enhance the treatment efficacy [24][34]; and suppress the effect of the transient increase of VEGF immediately after the occurrence of TACE-related ischemia, which is considered as one of the causes of tumor exacerbation [23][24][26][27][33,34,37,38]. Although multiple clinical trials investigating the efficacy of combination therapy with TACE and molecular-targeted drugs have been conducted, their efficacy has not been demonstrated. Under these circumstances, the TACTICS trial (TACE therapy in combination with sorafenib), which investigated the combined effect of sorafenib and lipiodol TACE, showed the potential effects of treatment with a combination of targeted drugs and TACE.
Sorafenib-TACE sequential therapy also extended the OS by 3.7 months compared with TACE alone (35.6 months vs. 31.9 months, HR: 0.924). These data indicate that sequential therapy with sorafenib and TACE was more effective than TACE alone in prolonging the PFS [28][40]. Although the TACTICS treatment improved the PFS, it did not significantly extend the OS. For this reason, 76.3% of the patients who received TACE alone also received post-trial treatment, and nearly half of these patients received sorafenib. Therefore, patients generally received aggressive post-trial treatment, which can extend the post-progression survival (PPS) and weaken the benefit obtained from the original trial treatment [29][41].
5. New Treatment Strategy for Intermediate Stage HCC: TACE plus Cancer Immunotherapy, and Conversion Therapy
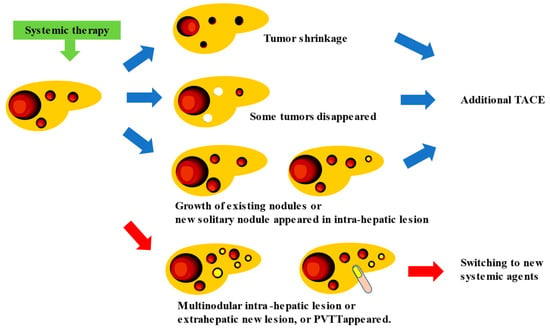
Currently, the development of new drugs for HCC focuses on establishing an effective cancer immunotherapy. With the advent of effective cancer immunotherapy, nivolumab monotherapy, pembrolizumab monotherapy, Atez/Bev combination therapy, and Dur/Tre combination therapy have been used as postoperative adjuvant chemotherapy for HCC, and their recurrence-suppressive effects after hepatic resection or ablation are being verified [30][44]. Atez/Bev combination therapy yielded a high response rate in patients with intermediate-stage HCC (44% ORR per RECIST v1.1) [8][31][32][8,45,46]. Unlike targeted agents, the use of Atez/Bev combination therapy resulted in a significant tumor shrinkage, even in patients with highly malignant positron emission tomography-positive HCC [33][47], including those with confluent multinodular and poorly differentiated HCC. Consequently, surgical excision, ablation, or curative TACE is feasible, resulting in the achievement of pathological CR and drug-free status in 20–30% of patients [33][47]. If systemic therapy is continued, the appropriate timing and method of TACE enforcement should be determined as no clear criteria have been established related to these aspects, that is, whether TACE should be performed “before PD occurs”, “what is the best response”, whether TACE should be administered in “nodules that showed PD”, whether TACE should be used “as conventional-TACE or DEB-TACE”, etc. This is because meta-analytic assessment is hampered by the excessive heterogeneity of intermediated-stage HCC between trials [34][48]. In addition, the progression pattern of patients treated with sorafenib influences the prognosis, and new vascular invasions or new extrahepatic lesions correlate with the worst prognosis [35][49]. This finding indicates that the prognosis differs depending on the type of PD caused by systemic therapy [22][31]. Therefore, the timing of TACE during continuous systemic therapy should be determined based on treatment responsiveness and type of PD assessed through radiological imaging. In intermediate-stage HCC, which has a variety of treatment patterns (whether TACE should be administered in combination with a single drug or performed three times, and whether the drugs should be changed to four or more), the appropriate treatment should be selected after considering the changes in liver function and tumor marker values. When systemic therapy is used in combination with TACE, systemic therapy with the same drug is continued, while TACE is only used for an existing tumor that shows slowed growth or for a new solitary nodule that appears as an intra-hepatic lesion even in patients with PD; when multiple tumor recurrences, distant metastasis, or vascular invasion occur, switching to new systemic therapeutic agents might be required (Figure 12) [33][36][47,50].