The role of oxidative stress (OS) in male infertility as a primary etiology and/or concomitant cause in other situations, such as inflammation, varicocele and gonadotoxin effects, is well documented. While reactive oxygen species (ROS) are implicated in many important roles, from spermatogenesis to fertilization, epigenetic mechanisms which are transmissible to offspring have also recently been described.
- male infertility
- spermatozoa
- oxidative stress
1. Introduction
Oxidative stress (OS) is associated with many risk factors for infertility, such as varicocele, inflammation, metabolic alterations, endogenous or exogenous toxins and radiofrequency [1][6]; however, it can also be observed as the only factor that causes unexplained infertility. Accordingly, the term MOSI (male-oxidative-stress-related infertility) indicates such a situation [2][7]. Since this mechanism is frequent, the administration of antioxidants has prevailed in clinical practice, usually without a previous evaluation of the effective presence of OS damage. Such an approach may be inappropriate, since the metaphor of a double-edged sword [1][3][6,8] should be considered when evaluating the physiological role and/or pathological effects of oxidants, often reported as reactive oxygen species (ROS).
2. Positive Effects of ROS
Surprisingly enough, cells such as spermatozoa, rich in substrates suitable for pre-venting oxidative stress and its perpetration, generate huge amounts of ROS. It is universally known that oxidative stress may impair the normal functioning of spermatozoa by reducing their motility and damaging their DNA through the direct and indirect oxidation of membrane lipids and nucleic acids. Despite this, spermatozoa are dependent from oxidative phosphorylation, which notably occurs in mitochondria and generates ROS because of electron leakage during the process. Moreover, spermatozoa usually have between 50 and 75 mitochondria, entirely located between the head and the flagellum. This leads to a significant production of ROS, among which the main species are represented by hydroxyl radicals (•OH), superoxide anion (•O2−), hydrogen peroxide (H2O2) and nitric oxide (NO) [19][24]. The central role of mitochondria is documented by the positive correlation between ROS generation by these organelles and sperm motility, with a consequent positive effect on pregnancy rate in vivo [20][25]. The compartmentalization of mitochondria in the mid-piece results in a physical separation from nuclear DNA, thus preventing possible damage in case of ROS overproduction. To balance the effect of ROS, spermatozoa are endowed with several antioxidant enzymes, albeit at a low concentration. Among these, superoxide dismutase, catalase and glutathione peroxidase are noteworthy; the former is involved in superoxide anion reduction while the latter two are mostly involved in hydrogen peroxide reduction [21][26]. The previously cited enzymes represent the intracellular side of antioxidant production. Apart from this, it is notable that seminal fluid itself is rich in antioxidants as well, both enzymatic and non-enzymatic; the total antioxidant power of seminal plasma was estimated to be 10 times higher than that of blood [22][27]. Among the antioxidants of the seminal plasma, some are worthy of mention: vitamin C, uric acid, glutathione, taurine and hypotaurine. A large part of the existing literature focuses on analyzing the effects of the exogenous administration of antioxidants, such as coenzyme Q10, L-acetyl-Carnitine, vitamin C and Zinc. The general consensus is that antioxidants counteract oxidative stress in semen and grant better results in terms of fertility; however, uncontrolled or excessive treatment with antioxidants may lead to an impairment in sperm function, which delineates a clinical entity known as reductive stress [23][28]. This observation suggests that sperm function rests on a thin edge between oxidation and reduction, and that spermatozoa are damaged by large amounts of oxidants, but also cannot properly function without it, which brings a original question: why do spermatozoa generate ROS at all? ROS have an established role as signal transductors in several cell types, but in spermatozoa they mediate the processes by which spermatozoa become capable of fertilizing: capacitation, hyperactivation and acrosome reaction. Moreover, ROS take part in the binding process with the oocyte, play a crucial role in compacting chromatin, and can modulate steroidogenesis. Capacitation is triggered in the female genital tract and consists of a series of metabolic and structural changes in spermatozoa that make them ready for fertilization. In short, superficial molecules present in the female genital tract strip “decapacitating factors” from the membrane of sperm cells, leading to rearrangement of membrane cholesterol and sphingolipids, inactivation of ATP-dependent Ca2+ regulatory channel (PMCA) and alkalinization of intracellular pH [24][29]. Moreover, Ca2+ is capable of activating a cytosolic adenylate cyclase, which in turn activates protein kinase A (PKA) through cAMP. The PKA then phosphorylates residues of Thr, Tyr and Ser, which activate a protein tyrosine kinase (PTK) and thus allow phosphorylation of more Tyr residues, signaling the activation of the sperm cell. Other signaling cascades, which are modulated by these phosphorylations, include ERK- and SRC (Rous Sarcoma oncogene)-mediated pathways [25][26][30,31]. The other role of PKA is to stimulate the NADPH oxidase to produce ROS; on the other hand, ROS are able to activate the cytosolic adenylate cyclase, thus amplifying this process, and by the inhibition of the action of phosphatases, limit its termination [27][32]. As a consequence of capacitation, spermatozoa have an alkaline intracellular pH and a hyperpolarized membrane, which leads to a rise in Ca2+ dismissal from the endoplasmic reticulum and, subsequently, an increase of Ca2+ admission from the extracellular side. This leads to enhanced cAMP generation from the intracellular adenylate cyclase ADCY10, which activates PKA and thus permits the phosphorylation of Ser, Thr and Tyr residues of dynein and axokinin in the axonema, consequently placing the flagellum in a hyperactivated state. Both cAMP and Ca2+ increase the rate and width of oscillations of the flagellum in a process defined as hyperactivation, which is crucial for spermatozoa to progress through the oviduct [28][34]. When spermatozoa encounter the cumulus surrounding the oocyte, their contact with the zona pellucida activates a transformation process known as acrosome reaction. In humans, contact with zona pellucida protein ZP3 seems to activate Ca2+ channels, which generate a transient Ca2+ increase in the sperm cell, which in turn allow for PI3K activation, generation of PIP3, activation of serine proteases PKB and PKCζ and ultimately exocytosis of molecules such as acrosin [29][37]. Acrosin is a serine protease which, among other things, helps to digest the membrane of spermatozoa, allowing the binding with the zona pellucida [27][32]. After acrosome reaction, a spermatozoon binds to the zona pellucida of the oocyte, allowing genetic material to be incorporated alongside that of the oocyte. The previous process requires the activation of Phospholipase A2 (PLA2), which rearranges membrane lipids and permits fusion between the membranes. Goldman et al. [30][39] showed that oxidants such as •O2− and H2O2 are capable of activating PKC, which in turn activates PLA2, thus participating in the sperm–oocyte binding. It is interesting to note that oocyte generates ROS as well after the binding of the spermatozoon to the zona pellucida. ROS production induces the phenomenon known as “zona hardening” in order to avoid polyspermy [31][40]. A decisive, yet often overlooked, role played by ROS in spermatozoa is the regulation of apoptosis. In fact, the regulation of apoptosis is fundamental in maintaining an adequate germ/Sertoli cell ratio during spermatogenesis, while at the same time eliminating defective sperm cells, determining an overall increase of fertility [32][44]. During cell life, apoptosis may occur via the intrinsic or the extrinsic pathway. Interestingly, spermatozoa seem to undergo apoptosis almost exclusively via the former [33][45]. Normally, ROS damage is responsible for lipid peroxidation of the mitochondria, membrane damage and activation of the caspase cascade [34][42]. In spermatozoa, however, mitochondria are strictly separated from the nucleus, and thus caspases cannot move to the head of the spermatozoon. The only molecule that can do so is H2O2, produced in the mitochondria and released from them during apoptosis. Hydrogen peroxide can then produce molecules such as acrolein and 4-HNE, which bind DNA, leading to its damage and, ultimately, cell death via apoptosis [35][46]. ROS appear to regulate this process through phosphorylation and de-phosphorylation of kinases such as PI3K, which in turn activates a cascade that leads to the phosphorylation of BCL2-associated death promoter (BAD), an important promoter of the caspase cascade, thus inactivating it [35][46]. The importance of phosphorylation and oxidation processes in orchestrating apoptosis has led to speculation about the nature of spermatozoa maturation. It has been hypothesized that capacitation is the same event that, through overproduction of ROS, leads to induction of apoptosis. After ejaculation, spermatozoa initiate capacitation shortly before their approach to the site of fertilization. If they do not achieve fertilization, ROS produced during capacitation ultimately overwhelm the defenses of the spermatozoa, leading to its apoptosis. Thus, paradoxically, the spermatozoa, the cells responsible for creating life, appear to actually be designed for death [36][47]. A summary of the main features is schematized in Figure 1.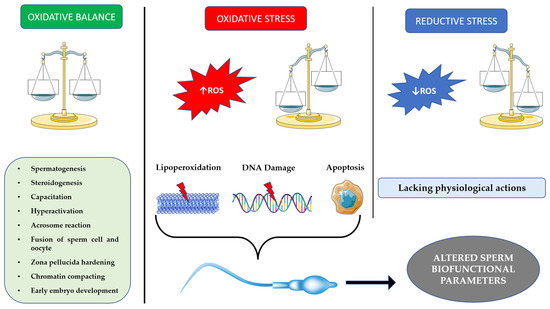
3. Vulnerability of Spermatozoa
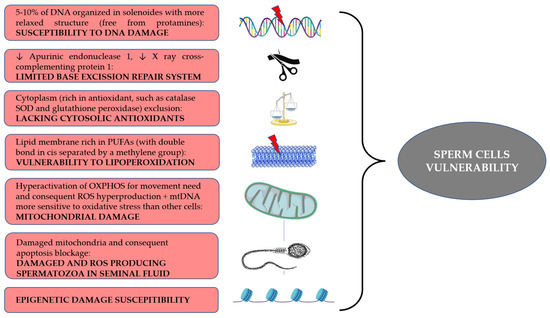
Spermatozoa are particularly vulnerable to oxidative damage due to multiple intrinsic structural characteristics of these specialized cells, but also due to the complex mechanism of differentiation (i.e., spermatogenesis) and maturation required to reach a full fertilizing capacity. During spermatogenesis, sperm chromatin undergoes many changes that lead to a tighter quaternary structure. After meiosis, histones are replaced with transition proteins, which in turn will be replaced with protamines type 1 and type 2. This allows the nucleus to be 6–7 times smaller than other cells’ nuclei. However, 5–10% of the sperm DNA are free from the binding with protamines. These portions are distributed in regions called “solenoids”, where they are bound with paternal nucleosome. These regions are more accessible to ROS due to their more relaxed structure, thus leading to DNA damage and possible fragmentation [37][48]. This represents the first step according to the “two-steps” hypothesis proposed by Aitken [38][49]. As for the second step, oxidative damage of DNA results in the generation of 8-desoxyguanosine (8OHdG) adducts. Spermatozoa are endowed with a limited base excision repair (BER) system. Although they possess an 8-oxoguanine glycosylase 1 (OGG1) enzyme, they lack the subsequent enzymes in the pathway, apurinic endonuclease 1 (APE1) and X-ray repair cross-complementing protein 1 (XRCC1). The former’s role is to cleave phosphate groups at the 3′ and 5′ of the baseless site, while the latter interacts with many enzymes in order to restore the normal DNA structure [39][50]. Thus, when an oxidative insult generates 8OHdG, OGG1 cleaves the adduct from DNA, generating an abasic site which cannot be further repaired by the spermatozoa, which either becomes totally dependent from the repairing system of the oocyte [32][44] or undergoes DNA fragmentation [40][51]. At the end of spermatogenesis, spermatozoa have expelled a large part of their cytoplasm. This means that cytosolic antioxidant systems are lacking as a result. Among the main antioxidants, catalase, superoxide dismutase (SOD) and glutathione peroxidase are noteworthy [38][49], but phospholipase A2 is also critical for its role in cleaving oxidized fatty acids, leading to their presentation to glutathione peroxidase. Finally, peroxiredoxins play an important role as well [41][52]. Peroxiredoxins are sulfydryl-dependent, selenium- and heme-free peroxidases, with high evolutionary conservation [42][53]. They play a role of importance in the oxidative milieu of the spermatozoa as they act as antioxidants, reducing highly reactive molecules such as H2O2 and in turn oxidizing thiol compounds. [41][52]. Thus, peroxiredoxins are an active part of H2O2 signaling. In particular, peroxiredoxin-2 (Prx2) has been found to transfer oxidative equivalents to compounds, coupled with scaffold protein STAT3 [12][17] and guided specifically to thiols by chaperone annexin A2 [43][54]. It has been shown that inhibitors of peroxiredoxins prevent capacitation in humans [44][55]. Oxidative stress may impair glutathione peroxidase activity also by inhibiting glucose-6-phostphate dehydrogenase, thus reducing the production of NADPH which is necessary to reduce oxidized glutathione [40][51]. On the other hand, excess of cytoplasm could be a factor of vulnerability as well, since its retention due to an altered maturation enhances enzymes related to ROS production, such as creatine kinase and glucose-6-phosphate dehydrogenase [45][56]. Spermatozoa have a plasma membrane rich in polyunsaturated fatty acids. These molecules are characterized by the presence of double bonds in cis configuration separated by methylene groups. Being close to a methylene group renders the bonds particularly vulnerable to oxidation. Lipid peroxidation (LPO) generates lipid radicals that propagate oxidation across the membrane. The process leads to the generation of by-products such as malondialdehyde, 4hydroxynonenal (4HNE) and acrolein. Malondialdehyde is mutagenic, but can also form DNA adducts, leading to mutations in tumor suppressor genes [32][44], while 4NHE and acrolein exert mainly a direct genotoxic effect on DNA [40][51] while also increasing ROS production in mitochondria [39][50].