You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Alessandro Allegra.
The rosemary plant, Rosmarinus officinalis L., one of the main members of the Lamiaceae family, is currently one of the most promising herbal medicines due to its pharmaceutical properties. Rosmarinic acid, beyond its anti-infectious, antioxidant, anti-inflammatory and immunomodulatory properties, has been extensively investigated in recent years for its anti-cancer activity on various apparently functionally disconnected molecular targets leading to various types of cancer.
- Rosmarinus officinalis
- rosemary
- skin
- cutaneous disease
- oxidative stress
1. Rosmarinus Officinalis and Antioxidant Action
Oxidative stress is the pathogenic primum movens of most cutaneous disorders, as the skin is the organ most widely and severely exposed to oxidative stress, despite the extensive endogenous and exogenous antioxidant system at its disposal [38][1]. For descriptive purposes, the causative agents of skin oxidative stress can be divided into exogenous and endogenous, including intracellular metabolic processes. The main exogenous pro-oxidant agents include ultraviolet (UV) light, environmental pollution and chronic psychological stress. The synergistic action of these factors accelerates the processes of pigmentation and skin aging [39][2]. The latter recognizes oxidative stress secondary to UV irradiation as the primary causal agent; hence, the need to coin the term photoaging. A greater contribution is made by UVA, since UVB, while participating in the damage, has limited penetration capacity into the epidermis and its cells, including keratinocytes and melanocytes above all [40][3]. The UV-induced oxidative damage of assorted intracellular structures can be direct or indirect. UVB, for the most part absorbed in the stratum corneum, is essentially accountable for the direct damage that sees DNA as its biologically most crucial molecular target [41][4]. Over the last two decades, the antioxidant potential of Rosmarinus officinalis and its bioactive constituents has been extensively investigated in both in vitro and in vivo studies, especially for its promising therapeutic effects on UV-induced photoaging, atopic dermatitis (AD) and pollution-induced skin aging. Relative to the aforementioned time frame, one of the first in vitro studies on this topic highlighted the interesting proportionality between protein glycation-inhibiting activity and antioxidant activity [42][5]. This functional synergy is said to be largely attributable to the polyphenolic compounds of various plant extracts, including Rosmarinus officinalis, paving the way for their prospective therapeutic use in diabetic complications and aging. Further confirmation of the antioxidant potential of this plant came from Ezzat et al., who emphasized the anti-wrinkle action of the defatted rosemary extract (DER), an effect attributable largely to rosmarinic acid, the main phenolic compound, but also to the diterpenes carnosic acid, carnosol and rosmanol [43][6]. Furthermore, the encapsulation of this extract in transferomes improves its skin penetrability. The most recent and relevant confirmation of the antioxidant action of rosemary’s phenolic compounds comes from an in vitro study evaluating the radical-scavenging and anti-aging activity of aqueous and ethanoic extracts of five phenolic-rich selected herbs, including Rosmarinus officinalis, which showed both the highest antioxidant activity and the most pronounced anti-elastase, anti-tyrosinase and anti-collagenase activity [44][7].
The recent literature is teeming with works confirming the photoprotective role of various plant extracts, of which rosemary, with its bioactive elements, is an increasingly consistent member. Hyuck Auh et al. pioneered the investigation of the anti-photoaging potential of combined extracts of marigold and rosemary, finding in a mouse model that the oral supplementation of these extracts suppressed UV-induced dermal–epidermal thickening in a dose-dependent manner; this histological finding was supported by reductions in various photoaging-related biomarkers observed in the lab [55][8]. A new molecular photoprotection mechanism was recently highlighted by Calniquer et al., who studied the phytotherapeutic efficacy of a combination of tomato and rosemary extracts. They found an interesting functional synergy of polyphenols (mainly represented by carnosic acid and carnosol in the rosemary extract) and carotenoids (mainly represented by lycopene, phytoene and phytofluene in the tomato extract) in activating the antioxidant response element/Nrf2 (ARE/Nrf2) transcription system, the main cellular antioxidant defense mechanism, in parallel with the inhibition of the UVB-induced pro-inflammatory nuclear factor kappa B (NFκB) pathway in keratinocytes and dermal fibroblasts, resulting in a decreased release of IL-6 and TNF-α and consequently a lowered activation of MMPs [56][9]. Additionally, for photoprotective purposes, the most recent strand of cosmetic research is working on the development of topical gel formulations containing Rosmarinus officinalis extract. The antioxidant, antiaging and healing potential of rosemary hexane extract was evaluated both in vitro and in a UVB-irradiated mouse model. Ibrahim et al. demonstrated the photoprotective potential of rosemary extract, the permeability and bioavailability of which improved when topically conveyed into lipid nanocapsule-based gel [57][10]. In the wake of the aforementioned work, Takayama et al. evaluated the in vitro antioxidant potential of rosemary hydroethanolic extract together with an evaluation, in vivo, of its anti-UVB photoprotective role if topically conveyed by an emulgel formulation [58][11]. The main findings regarding the role of Rosmarinus officinalis and its antioxidant functions are summarized in Table 1. The main antioxidant properties of Rosmarinus officinalis against UV-induced and pollution-induced skin aging and against cutaneous inflammation are shown in Figure 1.
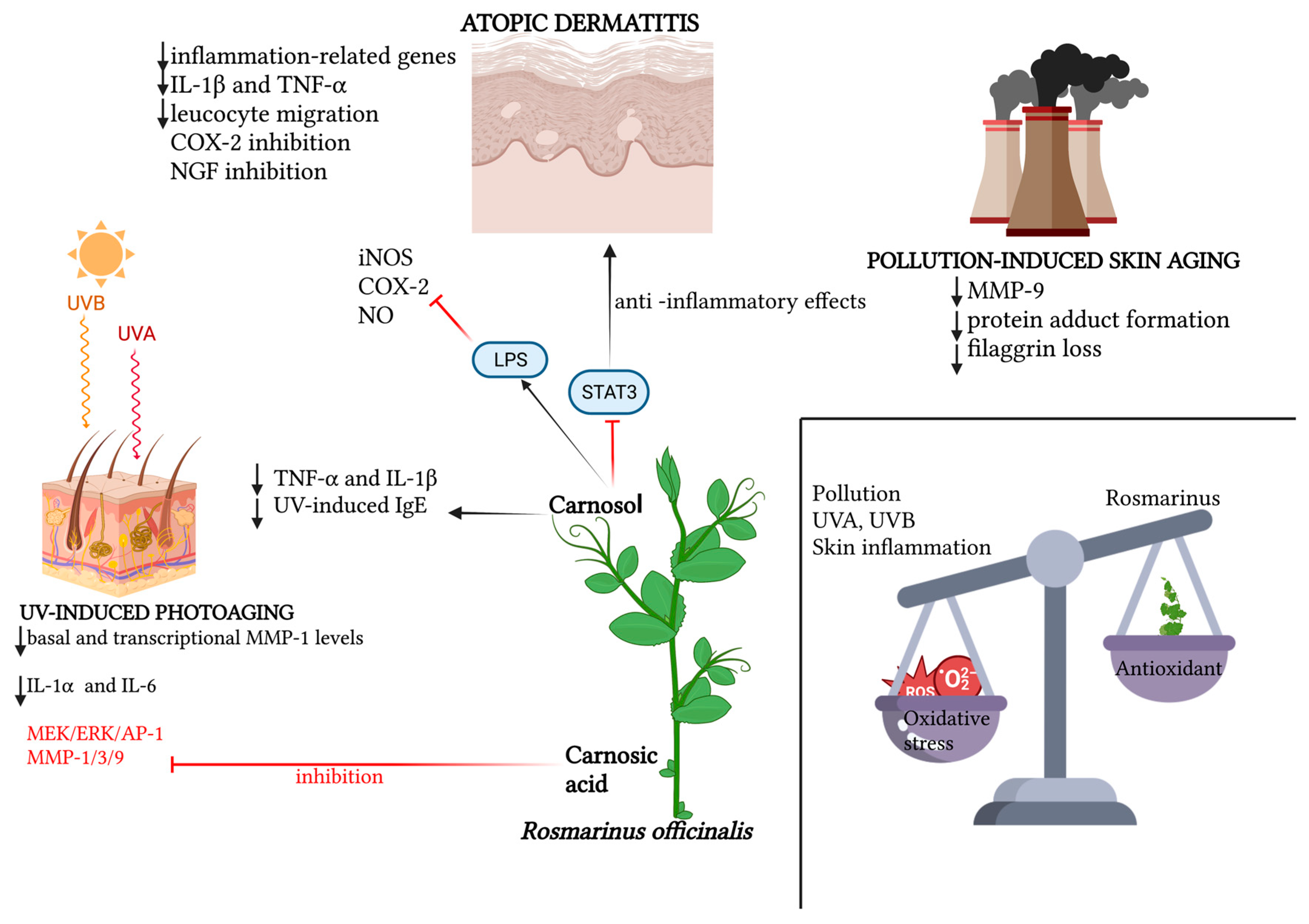
Figure 1. Schematic representation of the relevant molecular patterns involved in the promising antioxidant effects of Rosmarinus officinalis and its main bioactive compounds on three dermatological conditions: atopic dermatitis, UV-induced photoaging and pollution-induced skin aging. In atopic dermatitis, beyond the synergic action of carnosol and carnosic acid in downregulating inflammation-related genes, and therefore pro-inflammatory cytokines, leucocyte migration and NGF inhibition promotion, the specific action of carnosol on the STAT3 pathway is emphasized, for which the LPS-induced phosphorylation lock results in anti-inflammatory effects, together with carnosol’s direct inhibition of iNOS, NO and COX-2 activation. In UV-induced photoaging, the action of Rosmarinus officinalis in downregulating basal and transcriptional levels of MMP-1, as well as the inflammatory cytokines IL-6 and IL-1α, is highlighted. Also portrayed is the specific action of carnosol in downregulating TNF-α, IL-1β and serum levels of UV-induced IgE, and the inhibitory action of rosmarinic acid both on the MEK/ERK/AP-1 pathway and on MMP-1, MMP-3 and MMP-9. In pollution-induced skin aging, the downregulatory action of the two main phenolic diterpenes of Rosmarinus officinalis is mainly demonstrated on MMP-9, protein adduct formation and the loss of filaggrin. Created with BioRender.com.
Table 1.
The role of
Rosmarinus officinalis
against oxidative stress.
| Authors and Year | Topic | Model | Extraction Procedure | Study Characteristics |
|---|---|---|---|---|
| Takayama et al. [58][11], 2022 | Antioxidants and UVB protection | In vivo/in vitro | Exhaustive maceration | An in vitro and in vivo study on the properties of R. officinalis demonstrated its protective role for the skin against tissue damage caused by UVB radiation. |
| Nobile et al. [54][12], 2016 | Antioxidants and UVR protection | In vivo | Drying | The antioxidant, photoprotective and antiaging efficacy of the combination of Rosmarinus officinalis and Citrus paradisi extracts was demonstrated. |
| Ibrahim et al. [57][10], 2022 | Antioxidants and anti-aging | In vivo/in vitro | Not specified | The photoprotective potential of rosemary extract, whose permeability and bioavailability improved when topically conveyed into lipid nanocapsule-based gel, was assessed. |
| Nobile et al. [45][13], 2021 | Antioxidants and pollution | In vivo | Not specified | A double-blind randomized study demonstrated that oxidative stress-induced skin damage in both Asian and Caucasian women living in a polluted urban is reduced by oral supplementation with the following herbal extracts: Olea europaea leaf, Lippia citriodora, Rosmarinus officinalis, and Sophora japonica. |
| Mengoni et al. [47][14], 2011 | Antioxidants, inflammation and AD | In vivo/in vitro | Drying | In a mouse model, the expression of IL-1β and TNF-α, markers of inflammation-associated genes in skin, were reduced by carnosic acid and carnosol. |
| Calniquer et al. [56][9], 2021 | Antioxidants and UVB protection | In vitro | Not specified | An in vitro study demonstrated that the combination of carotenoids and polyphenols produces protective effects against UV-induced damage to skin cells, inhibiting UVB-induced NFκB activity and IL-6 release. |
| Sanchez et al. [53][15], 2014 | Antioxidants and UVB protection | In vivo/in vitro | Drying and water dissolution | In HaCaT keratinocytes and in human volunteers, the oral intake of rosemary and citrus bioflavonoid extracts reduced UVB-induced ROS, thus preventing cellular DNA damage. |
[39] and itraconazole-resistant Sporothrix brasiliensis [76,77][39][40]. The first attempt to assess the effectiveness of rosemary essential oil against cutaneous sporotrichosis in vivo was conducted by Waller et al. Itraconazole-resistant Sporothrix brasiliensis was inoculated into 30 Wistar mice, which were randomly treated with itraconazole, rosemary oil or saline as the control population. In mice treated with rosemary oil, the remission of skin lesions was noted, with mild to absent yeast cells. Furthermore, rosemary oil has also shown a protective effect on systemic organs, such as the liver and spleen, delaying the spread of infection [78][41]. Finally, rosemary has been evaluated for its antibacterial functions. It is known that bacterial pathogens have numerous virulent mechanisms allowing them to enter, replicate and persist at host sites, but with only a few common mechanisms. Among the possible alternatives to overcoming the issue of the constant increase in antibiotic resistance, inhibiting the virulence factors, which are responsible for the damage caused to the host tissue, is increasingly gaining ground as a new line of research [79][42]. By specifically inhibiting bacterial virulence mechanisms, the pathogenicity of bacteria could be controlled, thus avoiding the increasing ability of bacteria to develop antimicrobial resistance. Furthermore, selectively inhibiting virulence mechanisms reduces the risk of altering the composition of commensal microorganisms, which also play a beneficial role within and on the host. Quorum sensing, as a virulence mechanism, is a cell-density-dependent transcriptional regulatory system, used by bacteria to communicate and to adapt to the environment [80][43]. Staphylococcus aureus, a common cause of skin and soft tissue infection (SSTI), has generated increasing concern due to drug resistance. Staphylococcus aureus is an opportunistic Gram-positive bacterium, whose virulence mechanisms involve the activation of the quorum-sensing accessor gene regulator (agr) operon. The diterpene carnosic acid and carnosol, found in Rosmarinus officinalis L. leaves, have been demonstrated to have a specific inhibitory effect on Staphylococcus aureus agr expression, thus suppressing the cell–cell communication system and, consequently, its pathogenicity [80][43]. Finally, the activity of basil and rosemary essential oils has also been demonstrated against multi-resistant clinical strains of Escherichia coli. The results show that both essential oils tested were active against all clinical strains of Escherichia coli, including broad-spectrum β-lactamase-positive bacteria [81][44]. All of this evidence supports the idea that Rosmarinus officinalis can be used both as an important therapeutic tool and as an adjuvant within cosmetological formulations for its broad-spectrum antioxidant and antimicrobial capacities. Table 2 summarizes the main findings about the antimicrobial activity of Rosmarinus officinalis.
Table 2.
The antimicrobial activity of R. officinalis.
| Authors and Year | Topic | Model | Extraction Procedure | Study Characteristics |
|---|
. Similarly, Labib et al. highlighted in vivo the wound-healing potential of a combination of rosemary and tea tree essential oils incorporated into chitosan-based preparations. In the excision wound model in rats, their topical application resulted histologically in complete re-epithelialization associated with follicular activation, together with a significant increase in the rate of wound contraction. In addition, a marked reduction in oxidative stress in the wound area was highlighted, probably attributable to the antioxidant capacity of oxygenated monoterpenes, well-represented in both essential oils examined [87][50]. The most recent in vivo evidence on the wound-healing potential of rosemary highlights the anti-fungal role that bioactive compounds in its EO such as α-pinene might play, thereby speeding up the healing process [88][51].
Regarding the potential use of Rosmarinus officinalis in improving skin flap survival in relatively recent times, Ince et al. topically tested this ability in vivo with encouraging results [89][52]. These last were soon confirmed by the same author in another in vivo study that highlighted the vasodilatory effect of orally administered Rosmarinus officinalis oil. The resulting increased blood flow to the flap averted the dreaded necrotic complication, suggesting a systemic use of rosemary, especially in patients with circulatory disorders such as chronic obliterative artery disease [90][53]. In line with the future goals of the aforementioned work, in their latest in vivo study, Ince et al. identified two bioactive compounds from the essential oil of Rosmarinus officinalis, alpha-pinene and cineole, as the main systemic contributors to the increased flap survival [91][54]. Table 3 summarizes the main findings regarding the role of Rosmarinus officinalis in wound healing and skin flap survival.
Table 3.
The role of R. officinalis in wound healing and skin flap survival.
| Author | Topic | Model | Extraction Procedure | Study Characteristics | |||||
|---|---|---|---|---|---|---|---|---|---|
| Kallimanis et al. [73][36], 2022 | |||||||||
| Labib et al. [ | Anti-microbial activity | 87In vitro | After drying, the leaf was treated with each solvent in a 1:10 ratio, and then separated from the liquid part by filtration. | ][50Five different dry ROEs were assayed for their activities as antagonists of AhR ligand, which in turn inhibited Malassezia furfur yeasts. | |||||
| ], 2019 | Wound Healing | In vivo | Not specified | The wound-healing potential of a combination of rosemary and tea tree essential oils incorporated into chitosan-based preparations was highlighted. | De Macedo et al. [63][26], 2022 | Anti-microbial activity | In vitro | Maceration, infusion, Soxhlet and ultrasound | |
| Abu-Al-Basal et al. [84][47], 2010 | A topical formulation with R. officinalis extract demonstrated antimicrobial activity against | Wound healing | In vivo | S. aureus, S. oralis, | Steam distillation and P. aeruginosa | ||||
| An in vivo study conducted on BALB/c mice demonstrated the efficacy of both aqueous extract and essential oil of | Rosmarinus officinalis | in healing diabetic wounds. | Endo et al. [72][35], 2015 | Anti-microbial activity | In vitro | Leaf were dried in a circulating-air oven at 40 °C. Subsequently, they were soaked in 90/10% (v/v) ethanol–water for 48 h at 25 °C, protected from light. | Hydroalcoholic extracts from R. officinalis and T. riparia in vitro was demonstrated to have antifungal activity against strains of Trichophyton rubrum, Trichophyton mentagrophytes and Microsporum gypseum | ||
| Nakagawa et al. [80] | |||||||||
| Mekkaoui et al. [83][46], 2021 | Wound healing | In vivo | Not specified | A honey mixture with selected essential oils on chemical and thermal wound models in rabbits has healing effects. | [43], 2020 | Anti-microbial | In vitro | Not specified. | Diterpene carnosic acid and carnosol, present in Rosmarinus officinalis L. leaves, had specific effect on S. aureus agr expression. |
| Waller et al. [76] | |||||||||
| Sivamani et al. [85][48], 2012 | Wound healing | In silico |
98][61]. As already discussed, overexposure to UVB rays causes oxidative stress and DNA damage, resulting in an increased likelihood of developing different types of cutaneous cancer, including non-melanoma skin cancer and malignant melanoma [99,100][62][63]. ROS plays a pivotal role in oncogenesis and mutagenesis, especially in tumor promotion. ROS induces lipid peroxidation and DNA strand breaks by modulating different biochemical pathways and gene expression [101][64]. In recent years, increased scientific attention has been paid to identifying and characterizing natural compounds with chemopreventive properties against the formation of UVB-induced skin cancer [102][65].
A growing body of evidence indicates that specific compounds of rosemary, including carnosol, carnosic acid and rosmarinic acid, exert antiproliferative activity in several cancer cell lines [105,106,107,108][66][67][68][69]. In colorectal cancer cells, rosmarinic acid causes apoptosis [109][70], downregulating the mitogen-activated protein kinase (MAPK)/ERK pathway, while in hepatocellular carcinoma cells, rosemary essential oil reduced bcl-2 gene expression and upregulated bax gene expression [110][71]. In vivo, the anticancer properties of rosemary were proven in mice with acute myeloid leukemia, in which the increase in the administration of crude extracts of rosemary or carnosol, in combination with 1α-25 dihydroxy vitamin D3, led to an intense cytoprotective effect [111][72]. Thus, in vitro and in vivo data also indicated that crude extracts or purified components of rosemary exerted chemoprotective effects, inhibiting the early stages of tumor development [112[73][74],113], probably through the inhibition of enzymes of stage I carcinogenesis. Among the tumors with a rapidly increasing incidence rate, melanoma is a malignant tumor induced by the transformation of melanocytes [114][75]. When metastatic, the prognosis of melanoma becomes very bad, especially due to the poor response to the currently approved therapies. Hence, the growing interest in EOs is justified. On this topic, Huang et al. demonstrated in vitro that carnosol inhibited the migration of metastatic B16/F10 mouse melanoma cells through the suppression of MMP-9 expression. Furthermore, carnosol was shown to inhibit ERK1/2, AKT, p38 and c-Jun N-terminal kinases (JNK), and led to the activation of the transcription factors NFκB and c-Jun. From this assumption, the authors concluded that the invasive capacity of B16/F10 mouse melanoma cells could be limited by carnosol, through the downregulation of the above-mentioned pathways [115][76]. Finally, Cattaneo et al. highlighted that the proliferation of human melanoma cell line A375 was reduced by the hydroalcoholic extract of Rosmarinus officinalis, in a dose- and time-proportional way through cytotoxic and cytostatic effects on the cell cycle. Through the compositional characterization, the individual pure components of the extract were tested. The observations led researchers to hypothesize that the antiproliferative activity was a property of the entire extract, most likely deriving from multifactorial effects involving the majority of its elements [116][77]. All of these data agree in stating the potential role of rosemary in the therapy of various skin pathologies, first of all among skin cancer. From the analyzed studies, it emerges that the anti-cancer action derives in the first instance from its antioxidant action, which in its turn inhibits the genesis and progression of the tumor. Table 4 summarizes the main findings regarding the role of R. officinalis in cutaneous diseases.
Table 4.
The main findings regarding the role of
Rosmarinus officinalis
in cutaneous diseases.
| Author and Year | Topic | Model | Extraction Procedure | Study Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Panahi et al. [93][56], 2019 | Alopecia | Rosmarinus officinalis improved microcirculation surrounding the follicle, with comparable results to topical Minoxidil 2% in hair regrowth in patients affected by androgenetic alopecia. | ||||||||
| Rastegar et al. [94][57], 2013 | Alopecia | In vitro | The herbs were dried, crushed, and passed through 80-mesh stainless-steel sieves and water was used as a base. | Herbal extract with Rosmarinus officinalis and PRP had a positive effect on hair regrowth, promoting the proliferation of human dermal papilla. | ||||||
| Vagedes et al. [97][60], 2022 | Raynaud’s phenomenon | In vivo | Not specified. | In an open-label pilot study, warmth perception in patients with systemic sclerosis-related Raynaud’s phenomenon was increased by the application of topical rosemary essential oil. | ||||||
| Not specified | Rosmarinus, among other essential oils, inhibited the deleterious activities of elastase, thus ameliorating wound healing. | |||||||||
| Yimam et al. [98][61], 2017 | Cellulite | In vitro | Dried rosemary leaf was extracted with an approximately 10-fold volume of 95% ethyl alcohol at 40 °C. | A composition of extracts, including those from Rosmarinus officinalis, reduced lipid accumulation, platelet aggregation and inflammation, thus ameliorating microcirculation through antioxidant activity | [39], 2021 | |||||
| Sakhawy et al. [88] | Anti-microbial activity | [In vitro | Distillation by steam dragging in Clevenger equipment for 4 h | The study demonstrated rosemary oil as a promising antifungal to treat sporotrichosis, thus postponing systemic fungal spreading. | ||||||
| 51], 2023 | ||||||||||
| Tong et al. [103][78], 2018 | Wound healing | Non-Melanoma skin cancer | In vitro | Not specified. | Carnosol inhibits the UVB-induced activation of NF-κB, thus reducing keratinocyte carcinogenesis in vitro | Weckesser et al. [74][37], 2007 | Anti-microbial activity | In vitro | The solvent used was Carbon dioxide/isopropyl alcohol. | Rosmarinus extract inhibited the growth of Candida strains |
| Sancheti et al. [104][79], 2006 | Skin cancer | In vivo | Extraction in a Soxhlet apparatus with double-distilled water by refluxing for 36 h at 50–60 °C. | A mouse model demonstrated the protective role of Rosmarinus officinalis against skin tumorigenesis | Sienkiewicz et al. [81][44], 2013 | Anti-microbial activity | In vitro | Not specified. | ||
| Huang et al. [115 | Basil and | Rosmarinus officinalis | essential oils played a role against resistant | Escherichia coli clinical strains, and also against extended-spectrum β-lactamase positive bacteria. | ][ | Kim et al. [42][5], 2003 | ||||
| 76], 2005 | Melanoma | In vivo | Extraction with hexane, solvent evaporation, dissolving the dried material with methanol, and then filtrating and evaporating the solvent again. | Carnosol inhibited the migration of metastatic B16/F10 mouse melanoma cells in vitro by suppressing the expression of MMP-9 | Carbone et al. [71][34]Antioxidants | , 2013 | Anti-microbial activityIn vitro | In vitroEthanol/water (50:50, v/v) | The protein glycation inhibitory activity of aqueous ethanolic extracts of various plants, including Rosmarinus officinalis, closely correlated with the antioxidant activity of the extracts. | |
| Not specified. | ||||||||||
| Cattaneo et al. [116][77 | Nanostructured lipid carrier systems containing EOs, including | Rosmarinus officinalis | , could improve Clotrimazole effectiveness against candidiasis. | Salem et al. [44][7], 2020 | Antioxidants | In vitro | Drying, maceration, water distillation, boiling, filtration, lyophilization | An in vitro study evaluated the radical-scavenging and anti-aging activity of aqueous and ethanoic extracts of phenolic-rich selected herbs, including Rosmarinus officinalis, which showed the highest antioxidant activity and the most pronounced anti-elastase, anti-tyrosinase and anti-collagenase activity. | ||
| Ezzat et al. [43][6], 2016 | Antioxidants | In vivo/in vitro | Drying, pulverization, defatting, percolation with 70% ethanol, evaporation | The anti-wrinkle activity of DER, which was optimized by encapsulation in transferosomes, was assessed in an in vitro study. | ||||||
| Yeo et al. [50][16], 2019 | Antioxidants and atopic dermatitis | In vivo | Not specified | The anti-inflammation effect of the topical application of carnosol on UVB-induced skin inflammation in HR1 mice inhibited erythema, epidermal thickness and inflammatory responses. | ||||||
| Lee et al. [49 |
3. Rosmarinus Officinalis and Wound Healing
In the past decade, a new and interesting strand of phytotherapeutic research has become increasingly popular, which aims to evaluate the curative potential of Rosmarinus officinalis in wound healing, as well as in promoting the survival of skin flaps. Concerning the wound-healing process, beyond some in vivo evidence of the regenerative potential of Rosmarinus officinalis in the treatment of acute wounds, especially burn wounds, much of the scientific evidence instead concerns the therapeutic potential of rosemary in chronic wounds, primarily diabetic wounds [82,83][45][46].
First, Abu-Al-Basal, in an in vivo study conducted on BALB/c mice, demonstrated the efficacy of both aqueous extract and essential oil of Rosmarinus officinalis in healing diabetic wounds by pointing out the greater healing efficacy of the essential oil over the aqueous extract [84][47]. In the wake of this phytotherapeutic interest, Sivamani et al. evaluated the wound-healing role of EO components from various plants, identifying as a potential molecular therapeutic mechanism their ability to inhibit elastases produced by skin, neutrophils and germs, including Pseudomonas aeruginosa [85][48]. Additionally, Pérez-Recalde et al. confirmed the therapeutic potential of EOs of various plants, including rosemary, especially in chronic wounds. In rodent wounds, improved collagen deposition associated with increased fibroblastic proliferation and a faster wound closure rate has been observed, even highlighting the promising role in wound healing of the incorporation of EOs into resorbable polymeric scaffolds [86][49]
| In vivo | ||||||||
| Not specified | ||||||||
| Topical application of a mixture of essential oils, including | Rosmarinus officinalis | , had potential in healing wounds infected with | Candida albicans | . | ||||
| Farhan et al. [82][45], 2021 | Wound healing | In vivo | Methanol extraction | In vitro, the antifungal activities of Rosmarinus officinalis in wounds infected with Candida albicans was demonstrated. | ||||
| Ince et a [90][53], 2016 | Increasing skin flap survival | In vivo | Not specified | The vasodilatory effects of Rosmarinus officinalis contributed to increasing skin flap survival. | ||||
| Ince et al. [89][52], 2015 | ]Increasing skin flap survival | In vivo | Not specified. | Rosmarinus officinalis increased skin flap survival in a mouse model. | ||||
| , 2015 | Melanoma | In vitro | Grinding into fine powder and suspension at 330 g/L in a solution of 65% (w/ | Ince et al. [91][54], 2018 | Increasing skin flap survival | In vivo | Not specified | Alpha-pinene and cineole were the components of Rosmarinus officinalis responsible for increased flap survival. |
| ] | ||||||||
| [ | ||||||||
| 17], 2019 | Antioxidants and atopic dermatitis | In vivo/in vitro | Not specified | Carnosol inhibited LPS-induced nitric oxide generation and the expression of inflammatory marker proteins, including iNOS and COX-2 in RAW 264.7 cells. STAT3 phosphorylation and DNA-binding activity in RAW 264.7 cells were reduced. | ||||
| Takano et al. [48][18], 2011 | Antioxidant and atopic dermatitis | In vivo/in vitro | Ethanol | In an atopic dermatitis mouse model, the application of four herbal extracts, including Rosmarinus officinalis, reduced atopic lesions, thus inhibiting the effect of NGF on neuritic outgrowths in lesional skin. | ||||
| Martin et al. [51][19], 2008 | Anti UV | In vitro | Solubilization | IL1-α and IL-6, which play a role in the up-regulation of UV-induced MMP-1, could be suppressed by the Rosmarinus officinalis water-soluble extract. | ||||
| Park et al. [52][20], 2013 | Anti UV | In vitro | Not specified | The antiaging activity of carnosic acid downregulated the UV-induced expression of MMP-1, MMP-3 and MMP-9 in human fibroblasts and keratinocytes. | ||||
| Hoskin et al. [46][21], 2021 | Antioxidants and pollution | Ex vivo | Hydroalcoholization | The topical application of a gel based on hydroalcoholic rosemary extract complexed with algae proteins against pollution-induced oxidative skin damage was demonstrated. | ||||
| Hyuck Auh et al. [55][8], 2021 | Anti UV | In vivo | Ethanol at 72 °C for 3 h, evaporation | A mixture of marigold and rosemary extracts demonstrated anti-aging activity in a UV-induced mouse model of photoaging, with reduced expression of matrix metalloproteinase, interleukins, TNF-α, procollagen type I, superoxide dismutase, glutathione peroxidase and catalase |
2. Rosmarinus Officinalis and Antimicrobial Action
In recent times, the scientific literature has provided ever-increasing knowledge on the antimicrobial activities of essential oils, which are finding use in both medical and cosmetic fields. Secondary plant metabolites have a pharmacological effect on the treatment of skin disorders, for which they are used within topical formulations. In this regard, rosemary extract has shown antimicrobial activity in several cases [59,60,61,62][22][23][24][25]. Starting from the assumption that EOs possess antimicrobial activity, De Macedo et al. reported the first attempt to produce an oil-in-water emulsion containing only natural excipients and rosemary extract, demonstrating that higher quantities of extracted phenolic compounds, flavonoids and tannins corresponded to greater antioxidant and antimicrobial activity, especially against Staphylococcus aureus, Streptococcus oralis and Pseudomonas aeruginosa. De Macedo et al. concluded by asserting that this topical formulation based on Rosmarinus officinalis could be a natural therapeutic novelty against microorganisms, which are becoming increasingly resistant to conventional drugs [63][26]. Another field of research is the treatment of Candida species, which are developing increasing resistance to traditional drugs [64,65][27][28], including azoles and polyenes [66[29][30],67], thus representing a great challenge for the medical field in the treatment of such common skin infections. Furthermore, conventional antifungal drugs often exhibit toxic side effects for human cells, including hepatotoxic effects. Hence, there is growing scientific interest in EOs, natural compounds that are proving to be promising for their various antibacterial, antifungal and antiviral properties [68,69][31][32]. Their action against a wide variety of microorganisms is believed to be due to their ability to alter the membrane and the microorganisms’ cell wall, resulting in the extracellular loss of cytoplasmic material [70][33]. The pharmacological properties of EOs, mainly related to their complex chemical makeup and high levels of phenols, make these compounds a promising tool for the treatment and prevention of candidiasis [71][34]. However, the low solubility in water, high volatility and high instability of EOs represent the main limitations of their use in the pharmaceutical and cosmetic fields. To overcome this problem, the encapsulation of EOs has proven to be a useful solution. Starting from the knowledge that Rosmarinus officinalis can be successfully used as a matrix component and active ingredient of nanostructured lipid carriers (NLCs), it was subsequently demonstrated that the nanoparticles ensured a prolonged in vitro release of clotrimazole, thus increasing the antifungal activity. This confirmed that NLCs containing Mediterranean EOs represent a promising strategy to improve efficacy against topical candidiasis [71][34]. Specifically, an in vitro test highlighted that leaves from Rosmarinus officinalis and Tetradenia riparia contain antifungal bioactive compounds. Hydro-alcoholic extracts from these leaves seem to be effective against dermatophytes, including Trichophyton rubrum, Trichophyton mentagrophytes and Microsporum gypseum, with fungal growth inhibition and morphological alterations in the hyphae [72][35]. Moreover, it has been proven that the aryl hydrocarbon receptor (AhR), when activated by microbial metabolites, is implicated in a number of skin diseases. Starting from this assumption, Kallimanis et al. attempted to identify natural compounds potentially capable of inhibiting AhR activation by microbial ligands. In this regard, five different dry extracts of Rosmarinus officinalis were analyzed to evaluate its ability to inhibit AhR, confirming its dose-dependent antimicrobial activity against Malassezia furfur [73][36]. Another study conducted by Weckesser et al. evaluated the antimicrobial capacity of six extracts, including Rosmarinus officinalis, and plant extracts, including carnosol and carnosic acid, showing that Rosmarinus officinalis extract inhibited the growth of both aerobic and anaerobic bacteria and yeasts. Furthermore, the Rosmarinus extract was able to inhibit both Candida strains, further showing its antifungal action [74][37]. Sporotrichosis is a subcutaneous fungal infection caused by fungi of the genus Sporothrix, increasingly affecting humans and cats [75][38]. Despite the limited experimental data on the effects of rosemary essential oil on the treatment of sporotrichosis, studies in the current literature confirm its antifungal activity against Sporothrix schenckii [76]
4. Rosmarinus Officinalis and Cutaneous Diseases
Rosemary has been shown to have not only antioxidant and antimicrobial properties, but also a beneficial role in the treatment of various skin diseases.
In the treatment of alopecia aerate, an autoimmune disease affecting the follicles with subsequent hair loss, the essential oil of Rosmarinus officinalis managed to improve microcirculation surrounding the hair follicle [92][55]. Moreover, a clinical study compared the efficacy of rosemary essential oil to minoxidil 2% solution for the treatment of androgenetic alopecia. Patients used either minoxidil 2% solution or rosemary essential oil, with a dramatic increase in hair count reported for both treatments and without significant differences between the two study groups, which confirmed the therapeutic effectiveness of Rosmarinus officinalis. Moreover, scalp irritation was more frequent in the minoxidil 2% solution group, confirming the relatively few side effects of natural compound therapies [93][56]. Among the possible therapeutical approaches, increasing attention has been paid to platelet-rich plasma (PRP) to increase hair density and regrowth. The effect of PRP in combination with herbal extracts has been evaluated to identify the factors stimulating hair growth [94][57]. Combined herbal extracts and PRP promoted the proliferation of human dermal papilla cells via the regulation of extracellular signal-regulated kinase (ERK) and protein kinase B (Akt) proteins, shedding light on the possible future development of herbal extracts and PRP combination therapies in order to enhance hair growth. The role of Rosmarinus officinalis has also been evaluated in systemic sclerosis-related Raynaud’s. Additionally, in this connective tissue disorder, the high levels of ROS contribute to the development of fibrotic processes and closely correlate with the severity of skin fibrosis [95,96][58][59]. In an open-label pilot study, Vagedes et al. enrolled twelve patients, each of whom received an application of olive oil on both hands as a control and three hours later an application of 10% essential oil of Rosmarinus officinalis L., highlighting that warmth perception in patients with Raynaud’s phenomenon was ameliorated by topical rosemary EO application [97][60]. The potential role of rosemary has also been evaluated from an aesthetic perspective, specifically in the treatment of cellulite, a condition characterized by localized adiposity and inflammation, with subsequent alteration of the microcirculation, mostly affecting women. On this topic, in 3T3-L1 cells (a mouse cell line with an adipocyte-like phenotype), a composition of extracts, including those from Rosmarinus officinalis, was shown to reduce lipid accumulation, platelet aggregation and inflammation, thus ameliorating microcirculation through a dose-dependent inhibition of free radical formation. This evidence suggests the potential topical use of rosemary, combined with other extracts, and also in the aesthetic field, taking advantage of its anti-inflammatory and antioxidant properties [
| w |
| ) ethanol/water for 21 days. The extract was then filtered and stored at −20 °C until use. |
| In vitro, extract of |
| Rosmarinus officinalis L |
| . inhibited human melanoma A375 cell line proliferation in a dose- and time-proportional way |
5. Rosmarinus Officinalis and Cutaneous Lymphoma
Other skin disorders, including lymphomas, may benefit from the antioxidant properties of rosemary. A rare and frequently severe T-cell lymphoma, which can develop in the blood, lymph nodes or skin, is known as adult T-cell leukemia/lymphoma (ATLL). Human T-cell lymphotropic virus type 1 (HTLV-1) infection has been related to ATLL onset; however, less than 5% of HTLV-1 infected-patients develop ATLL. The Caribbean, some regions of South and Central America, and some portions of Africa are the areas where the HTLV-1 virus is most prevalent. Through their crucial functions in accelerating cell proliferation and preventing cell death, the viral genes tax and HTLV-1 bZIP factor (HBZ) supports the growth of infected cells. An ATL clone emerged as a result of the persistence of infected clones in vivo and the accumulation of genetic mutations and abnormal epigenetic alterations in host genes [117][80]. According to a study, the viral oncoproteins Tax and HBZ generate oxidative stress, mitochondrial damage and cytotoxicity, which are countered by the TP53-induced glycolysis and apoptosis regulator (TIGAR), which in turn is induced by the HTLV-1 latency-maintenance factor p30II. In colony transformation and foci formation assays, the p30II protein works in concert with Tax and HBZ to increase their oncogenic potential [118][81]. Additionally, in an in vivo xenograft model of HTLV-1-induced T-cell lymphoma, the authors demonstrated that TIGAR is substantially expressed in HTLV-1-induced tumors linked to oncogene deregulation and enhanced angiogenesis. These results show that the key oncoproteins Tax and HBZ likely work together as cofactors during retroviral carcinogenesis [119][82]. Therefore, reducing oxidative stress could alter the proliferative dynamics in ATLL patients. An experimental study revealed that carnosol caused ATL cell apoptosis through the inhibition of cell proliferation. The authors then used mass spectrometry and proteome analysis with fluorescent two-dimensional differential gel electrophoresis to look into the apoptosis-inducing mechanism of carnosol. According to the proteome study, carnosol-treated cells expressed more reductases, glycolytic pathway enzymes and enzymes in the pentose phosphate pathway than untreated cells did. These findings suggest that carnosol had an impact on the cell redox state. Additionally, the quantitative examination of glutathione, which is crucial for maintaining the intracellular redox state, revealed that carnosol was the reason for the decreased glutathione levels in cells. Furthermore, N-acetyl-L-cysteine, which is the precursor of glutathione, reduced carnosol efficiency. From these findings, it was suggested that the apoptosis-inducing activity of carnosol in ATL cells was provoked by the depletion of glutathione [120][83]. Although the results in the literature on the relationship between Rosmarinus officinalis and cutaneous lymphomas are rather limited, in vitro studies would seem to confirm the antineoplastic activity of this substance. Visanji et al. studied the antiproliferative effects of carnosol and carnosic acid on Caco-2 cells, demonstrating that after incubation with these components, the cells increased their doubling time, i.e., the time required to double their population. This was estimated to be due to G2/M phase cell cycle arrest. Furthermore, carnosic acid and carnosol were observed to arrest the cell cycle at different times. While carnosic acid arrested cells before prometaphase by reducing cyclin A levels, carnosol exerted its major impact on the cell cycle after prometaphase [121][84]. All of these data could provide the basis not only for an investigation of the potential chemopreventive role of Rosmarinus through cell cycle arrest, but also for an evaluation of the existence of the possible synergistic action of rosemary with traditional chemotherapeutic drugs, to assess the possibility that it can reduce the evolution of viral infection to neoplastic disease.
References
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575.
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative Stress in the Skin: Impact and Related Protection. Int. J. Cosmet. Sci. 2021, 43, 495–509.
- Rinnerthaler, M.; Bischof, J.; Streubel, M.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589.
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29.
- Kim, H.Y.; Kim, K. Protein Glycation Inhibitory and Antioxidative Activities of Some Plant Extracts in vitro. J. Agric. Food Chem. 2003, 51, 1586–1591.
- Ezzat, S.M.; Salama, M.M.; ElMeshad, A.N.; Teaima, M.H.; Rashad, L.A. HPLC–DAD–MS/MS Profiling of Standardized Rosemary Extract and Enhancement of Its Anti-Wrinkle Activity by Encapsulation in Elastic Nanovesicles. Arch. Pharm. Res. 2016, 39, 912–925.
- Salem, M.A.; Radwan, R.A.; Mostafa, E.S.; Alseekh, S.; Fernie, A.R.; Ezzat, S.M. Using an UPLC/MS-Based Untargeted Metabolomics Approach for Assessing the Antioxidant Capacity and Anti-Aging Potential of Selected Herbs. RSC Adv. 2020, 10, 31511–31524.
- Auh, J.-H.; Madhavan, J. Protective Effect of a Mixture of Marigold and Rosemary Extracts on UV-Induced Photoaging in Mice. Biomed. Pharmacother. 2021, 135, 111178.
- Calniquer, G.; Khanin, M.; Ovadia, H.; Linnewiel-Hermoni, K.; Stepensky, D.; Trachtenberg, A.; Sedlov, T.; Braverman, O.; Levy, J.; Sharoni, Y. Combined Effects of Carotenoids and Polyphenols in Balancing the Response of Skin Cells to UV Irradiation. Molecules 2021, 26, 1931.
- Ibrahim, N.; Abbas, H.; El-Sayed, N.S.; Gad, H.A. Rosmarinus officinalis L. Hexane Extract: Phytochemical Analysis, Nanoencapsulation, and in silico, in vitro, and in vivo Anti-Photoaging Potential Evaluation. Sci. Rep. 2022, 12, 13102.
- Takayama, K.S.; Monteiro, M.C.; Saito, P.; Pinto, I.C.; Nakano, C.T.; Martinez, R.M.; Thomaz, D.V.; Verri JR, W.A.; Baracat, M.M.; Arakawa, N.S.; et al. Rosmarinus officinalis Extract-Loaded Emulgel Prevents UVB Irradiation Damage to the Skin. An. Acad. Bras. Cienc. 2022, 94.
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin Photoprotective and Antiageing Effects of a Combination of Rosemary (Rosmarinus officinalis) and Grapefruit (Citrus paradisi) Polyphenols. Food Nutr. Res. 2016, 60, 31871.
- Nobile, V.; Schiano, I.; Peral, A.; Giardina, S.; Spartà, E.; Caturla, N. Antioxidant and Reduced Skin-Ageing Effects of a Polyphenol-Enriched Dietary Supplement in Response to Air Pollution: A Randomized, Double-Blind, Placebo-Controlled Study. Food Nutr. Res. 2021, 65.
- Mengoni, E.S.; Vichera, G.; Rigano, L.A.; Rodriguez-Puebla, M.L.; Galliano, S.R.; Cafferata, E.E.; Pivetta, O.H.; Moreno, S.; Vojnov, A.A. Suppression of COX-2, IL-1β and TNF-α Expression and Leukocyte Infiltration in Inflamed Skin by Bioactive Compounds from Rosmarinus officinalis L. Fitoterapia 2011, 82, 414–421.
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Alcaraz, M.; Micol, V. Protective Effects of Citrus and Rosemary Extracts on UV-Induced Damage in Skin Cell Model and Human Volunteers. J. Photochem. Photobiol. B 2014, 136, 12–18.
- Yeo, I.J.; Park, J.H.; Jang, J.S.; Lee, D.Y.; Park, J.E.; Choi, Y.E.; Joo, J.H.; Song, J.K.; Jeon, H.O.; Hong, J.T. Inhibitory Effect of Carnosol on UVB-Induced Inflammation via Inhibition of STAT3. Arch. Pharm. Res. 2019, 42, 274–283.
- Lee, D.Y.; Hwang, C.J.; Choi, J.Y.; Park, M.H.; Song, M.J.; Oh, K.W.; Son, D.J.; Lee, S.H.; Han, S.B.; Hong, J.T. Inhibitory Effect of Carnosol on Phthalic Anhydride-Induced Atopic Dermatitis via Inhibition of STAT3. Biomol. Ther. 2017, 25, 535–544.
- Takano, N.; Inokuchi, Y.; Kurachi, M. Effects of Ethanol Extracts of Herbal Medicines on Dermatitis in an Atopic Dermatitis Mouse Model. Yakugaku Zasshi 2011, 131, 581–586.
- Martin, R.; Pierrard, C.; Lejeune, F.; Hilaire, P.; Breton, L.; Bernerd, F. Photoprotective Effect of a Water-Soluble Extract of Rosmarinus officinalis L. against UV-Induced Matrix Metalloproteinase-1 in Human Dermal Fibroblasts and Reconstructed Skin. Eur. J. Dermatol. 2008, 18, 128–135.
- Park, M.; Han, J.; Lee, C.S.; Heung Soo, B.; Lim, K.-M.; Ha, H. Carnosic Acid, a Phenolic Diterpene from Rosemary, Prevents UV-Induced Expression of Matrix Metalloproteinases in Human Skin Fibroblasts and Keratinocytes. Exp. Dermatol. 2013, 22, 336–341.
- Hoskin, R.; Pambianchi, E.; Pecorelli, A.; Grace, M.; Therrien, J.-P.; Valacchi, G.; Lila, M.A. Novel Spray Dried Algae-Rosemary Particles Attenuate Pollution-Induced Skin Damage. Molecules 2021, 26, 3781.
- Bernardes, W.A.; Lucarini, R.; Tozatti, M.G.; Souza, M.G.M.; Andrade Silva, M.L.; da Silva Filho, A.A.; Martins, C.H.G.; Miller Crotti, A.E.; Pauletti, P.M.; Groppo, M.; et al. Antimicrobial Activity of Rosmarinus officinalis against Oral Pathogens: Relevance of Carnosic Acid and Carnosol. Chem. Biodivers. 2010, 7, 1835–1840.
- Zhong, X.; Wang, X.; Zhou, N.; Li, J.; Liu, J.; Yue, J.; Hao, X.; Gan, M.; Lin, P.; Shang, X. Chemical Characterization of the Polar Antibacterial Fraction of the Ethanol Extract from Rosmarinus Officinalis. Food Chem. 2021, 344, 128674.
- de Paula, I.M.B.; Moraes, F.C.; de Souza, O.V.; Yamamoto, C.H. Development of Mouthwash with Rosmarinus officinalis Extract. Braz. J. Pharm. Sci. 2014, 50, 851–858.
- Karadağ, A.E.; Demirci, B.; Çaşkurlu, A.; Demirci, F.; Okur, M.E.; Orak, D.; Sipahi, H.; Başer, K.H.C. In Vitro Antibacterial, Antioxidant, Anti-Inflammatory and Analgesic Evaluation of Rosmarinus officinalis L. Flower Extract Fractions. S. Afr. J. Bot. 2019, 125, 214–220.
- Macedo, L.M.d.; Santos, É.M.d.; Ataide, J.A.; Silva, G.T.d.S.e.; Guarnieri, J.P.d.O.; Lancellotti, M.; Jozala, A.F.; Rosa, P.C.P.; Mazzola, P.G. Development and Evaluation of an Antimicrobial Formulation Containing Rosmarinus Officinalis. Molecules 2022, 27, 5049.
- Pfaller, M.A.; Diekema, D.J. Rare and Emerging Opportunistic Fungal Pathogens: Concern for Resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 2004, 42, 4419–4431.
- Costa, C.; Ribeiro, J.; Miranda, I.M.; Silva-Dias, A.; Cavalheiro, M.; Costa-de-Oliveira, S.; Rodrigues, A.G.; Teixeira, M.C. Clotrimazole Drug Resistance in Candida Glabrata Clinical Isolates Correlates with Increased Expression of the Drug:H+ Antiporters CgAqr1, CgTpo1_1, CgTpo3, and CgQdr2. Front. Microbiol. 2016, 7, 526.
- Williams, D.W.; Kuriyama, T.; Silva, S.; Malic, S.; Lewis, M.A.O. Candida Biofilms and Oral Candidosis: Treatment and Prevention. Periodontology 2011, 55, 250–265.
- Ben-Ami, R. Treatment of Invasive Candidiasis: A Narrative Review. J. Fungi 2018, 4, 97.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475.
- Bona, E.; Cantamessa, S.; Pavan, M.; Novello, G.; Massa, N.; Rocchetti, A.; Berta, G.; Gamalero, E. Sensitivity of Candida albicans to Essential Oils: Are They an Alternative to Antifungal Agents? J. Appl. Microbiol. 2016, 121, 1530–1545.
- Altintas, A.; Tabanca, N.; Tyihák, E.; Ott, P.G.; Móricz, Á.M.; Mincsovics, E.; Wedge, D.E. Characterization of Volatile Constituents from Origanum Onites and Their Antifungal and Antibacterial Activity. J. AOAC Int. 2013, 96, 1200–1208.
- Carbone, C.; do Teixeira, M.C.; do Sousa, M.C.; Martins-Gomes, C.; Silva, A.M.; Souto, E.M.B.; Musumeci, T. Clotrimazole-Loaded Mediterranean Essential Oils NLC: A Synergic Treatment of Candida Skin Infections. Pharmaceutics 2019, 11, 231.
- Endo, E.H.; Costa, G.M.; Nakamura, T.U.; Nakamura, C.V.; Dias Filho, B.P. Antidermatophytic Activity of Hydroalcoholic Extracts from Rosmarinus officinalis and Tetradenia Riparia. J. Mycol. Med. 2015, 25, 274–279.
- Kallimanis, P.; Chinou, I.; Panagiotopoulou, A.; Soshilov, A.A.; He, G.; Denison, M.S.; Magiatis, P. Rosmarinus officinalis L. Leaf Extracts and Their Metabolites Inhibit the Aryl Hydrocarbon Receptor (AhR) Activation in vitro and in Human Keratinocytes: Potential Impact on Inflammatory Skin Diseases and Skin Cancer. Molecules 2022, 27, 2499.
- Weckesser, S.; Engel, K.; Simon-Haarhaus, B.; Wittmer, A.; Pelz, K.; Schempp, C.M. Screening of Plant Extracts for Antimicrobial Activity against Bacteria and Yeasts with Dermatological Relevance. Phytomedicine 2007, 14, 508–516.
- Morgado, D.S.; Castro, R.; Ribeiro-Alves, M.; Corrêa-Moreira, D.; Castro-Alves, J.; Pereira, S.A.; Menezes, R.C.; Oliveira, M.M.E. Global Distribution of Animal Sporotrichosis: A Systematic Review of Sporothrix sp. Identified Using Molecular Tools. Curr. Res. Microb. Sci. 2022, 3, 100140.
- Waller, S.B.; Madrid, I.M.; Cleff, M.B.; Santin, R.; Freitag, R.A.; Meireles, M.C.A.; Mello, J.R.B. Effects of Essential Oils of Rosmarinus officinalis Linn. and Origanum vulgare Linn. from Different Origins on Sporothrix Brasiliensis and Sporothrix Schenckii Complex. Arq. Bras. Med. Vet. Zootec. 2016, 68, 991–999.
- Waller, S.B.; Madrid, I.M.; Silva, A.L.; Dias de Castro, L.L.; Cleff, M.B.; Ferraz, V.; Meireles, M.C.A.; Zanette, R.; de Mello, J.R.B. In Vitro Susceptibility of Sporothrix Brasiliensis to Essential Oils of Lamiaceae Family. Mycopathologia 2016, 181, 857–863.
- Waller, S.B.; Cleff, M.B.; Dalla Lana, D.F.; de Mattos, C.B.; Guterres, K.A.; Freitag, R.A.; Sallis, E.S.V.; Fuentefria, A.M.; de Mello, J.R.B.; de Faria, R.O.; et al. Can the Essential Oil of Rosemary (Rosmarinus officinalis Linn.) Protect Rats Infected with Itraconazole-Resistant Sporothrix Brasiliensis from Fungal Spread? J. Med. Mycol. 2021, 31, 101199.
- Le, K.Y.; Otto, M. Quorum-Sensing Regulation in Staphylococci—An Overview. Front. Microbiol. 2015, 6, 1174.
- Nakagawa, S.; Hillebrand, G.G.; Nunez, G. Rosmarinus officinalis L. (Rosemary) Extracts Containing Carnosic Acid and Carnosol Are Potent Quorum Sensing Inhibitors of Staphylococcus Aureus Virulence. Antibiotics 2020, 9, 149.
- Sienkiewicz, M.; Łysakowska, M.; Pastuszka, M.; Bienias, W.; Kowalczyk, E. The Potential of Use Basil and Rosemary Essential Oils as Effective Antibacterial Agents. Molecules 2013, 18, 9334–9351.
- Farhan, A.; Alsuwayt, B.; Alanazi, F.; Yaseen, A.; Ashour, M.A. Evaluation and HPLC Characterisation of a New Herbal Ointment for the Treatment of Full-Thickness Burns in Rats. J. Taibah Univ. Med. Sci. 2021, 16, 152–161.
- Mekkaoui, M.; Assaggaf, H.; Qasem, A.; El-Shemi, A.; Abdallah, E.M.; Bouidida, E.H.; Naceiri Mrabti, H.; Cherrah, Y.; Alaoui, K. Ethnopharmacological Survey and Comparative Study of the Healing Activity of Moroccan Thyme Honey and Its Mixture with Selected Essential Oils on Two Types of Wounds on Albino Rabbits. Foods 2021, 11, 28.
- Abu-Al-Basal, M.A. Healing Potential of Rosmarinus officinalis L. on Full-Thickness Excision Cutaneous Wounds in Alloxan-Induced-Diabetic BALB/c Mice. J. Ethnopharmacol. 2010, 131, 443–450.
- Sivamani, P.; Singaravelu, G.; Thiagarajan, V.; Jayalakshmi, T.; Kumar, G.R. Comparative Molecular Docking Analysis of Essential Oil Constituents as Elastase Inhibitors. Bioinformation 2012, 8, 457–460.
- Pérez-Recalde, M.; Ruiz Arias, I.E.; Hermida, É.B. Could Essential Oils Enhance Biopolymers Performance for Wound Healing? A Systematic Review. Phytomedicine 2018, 38, 57–65.
- Labib, R.M.; Ayoub, I.M.; Michel, H.E.; Mehanny, M.; Kamil, V.; Hany, M.; Magdy, M.; Moataz, A.; Maged, B.; Mohamed, A. Appraisal on the Wound Healing Potential of Melaleuca Alternifolia and Rosmarinus officinalis L. Essential Oil-Loaded Chitosan Topical Preparations. PLoS ONE 2019, 14, e0219561.
- El-Sakhawy, M.A.; Soliman, G.A.; El-Sheikh, H.H.; Ganaie, M.A. Anticandidal Effect of Eucalyptus Oil and Three Isolated Compounds on Cutaneous Wound Healing in Rats. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 26–37.
- Ince, B.; Yildirim, A.M.; Okur, M.I.; Dadaci, M.; Yoruk, E. Effects of Rosmarinus officinalis on the Survivability of Random-Patterned Skin Flaps: An Experimental Study. J. Plast. Surg. Hand Surg. 2015, 49, 83–87.
- Ince, B.; Bilgen, F.; Gundeslioglu, A.O.; Dadaci, M.; Kozacioglu, S. Use of Systemic Rosmarinus officinalis to Enhance the Survival of Random-Pattern Skin Flaps. Balk. Med. J. 2016, 33, 645–651.
- Ince, B.; Dadaci, M.; Kilinc, I.; Oltulu, P.; Yarar, S.; Uyar, M. Effect of Cineole, Alpha-Pinene, and Camphor on Survivability of Skin Flaps. Turk. J. Med. Sci. 2018, 48, 644–652.
- Maleš, Ž.; Drvar, D.L.; Duka, I.; Žužul, K. Application of Medicinal Plants in Several Dermatovenerological Entities. Acta Pharm. 2019, 69, 525–531.
- Panahi, Y.; Taghizadeh, M.; Marzony, E.T.; Sahebkar, A. Rosemary Oil vs. Minoxidil 2% for the Treatment of Androgenetic Alopecia: A Randomized Comparative Trial. Skinmed 2015, 13, 15–21.
- Rastegar, H.; Ahmadi Ashtiani, H.; Aghaei, M.; Ehsani, A.; Barikbin, B. Combination of Herbal Extracts and Platelet-Rich Plasma Induced Dermal Papilla Cell Proliferation: Involvement of ERK and Akt Pathways. J. Cosmet. Derm. 2013, 12, 116–122.
- Piera-Velazquez, S.; Jimenez, S.A. Oxidative Stress Induced by Reactive Oxygen Species (ROS) and NADPH Oxidase 4 (NOX4) in the Pathogenesis of the Fibrotic Process in Systemic Sclerosis: A Promising Therapeutic Target. J. Clin. Med. 2021, 10, 4791.
- Bagnato, G.L.; Irrera, N.; Pizzino, G.; Santoro, D.; Roberts, W.N.; Bagnato, G.; Pallio, G.; Vaccaro, M.; Squadrito, F.; Saitta, A.; et al. Dual Avβ3 and Avβ5 Blockade Attenuates Fibrotic and Vascular Alterations in a Murine Model of Systemic Sclerosis. Clin. Sci. 2018, 132, 231–242.
- Vagedes, J.; Henes, J.; Deckers, B.; Vagedes, K.; Kuderer, S.; Helmert, E.; von Schoen-Angerer, T. Topical Rosmarinus officinalis L. in Systemic Sclerosis-Related Raynaud’s Phenomenon: An Open-Label Pilot Study. Complement. Med. Res. 2022, 29, 242–248.
- Yimam, M.; Lee, Y.-C.; Jiao, P.; Hong, M.; Brownell, L.; Jia, Q. A Standardized Composition Comprised of Extracts from Rosmarinus Officinalis, Annona Squamosa and Zanthoxylum Clava-Herculis for Cellulite. Pharmacogn. Res. 2017, 9, 319.
- Jhappan, C.; Noonan, F.P.; Merlino, G. Ultraviolet Radiation and Cutaneous Malignant Melanoma. Oncogene 2003, 22, 3099–3112.
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-Induced Skin Damage. Toxicology 2003, 189, 21–39.
- Borgia, F.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative Stress and Phototherapy in Atopic Dermatitis: Mechanisms, Role, and Future Perspectives. Biomolecules 2022, 12, 1904.
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739.
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical Profile of Rosmarinus officinalis and Salvia Officinalis Extracts and Correlation to Their Antioxidant and Anti-Proliferative Activity. Food Chem. 2013, 136, 120–129.
- Cheung, S.; Tai, J. Anti-Proliferative and Antioxidant Properties of Rosemary Rosmarinus Officinalis. Oncol. Rep. 2007, 17, 1525–1531.
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory Effects of Rosemary Extracts, Carnosic Acid and Rosmarinic Acid on the Growth of Various Human Cancer Cell Lines. Plant Foods Hum. Nutr. 2010, 65, 158–163.
- Johnson, J.J. Carnosol: A Promising Anti-Cancer and Anti-Inflammatory Agent. Cancer Lett. 2011, 305, 1–7.
- Xavier, C.P.R.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Salvia fruticosa, Salvia officinalis, and Rosmarinic Acid Induce Apoptosis and Inhibit Proliferation of Human Colorectal Cell Lines: The Role in MAPK/ERK Pathway. Nutr. Cancer 2009, 61, 564–571.
- Wei, F.-X.; Liu, J.-X.; Wang, L.; Li, H.-Z.; Luo, J.-B. Expression of Bcl-2 and Bax Genes in the Liver Cancer Cell Line HepG2 after Apoptosis Induced by Essential Oils from Rosmarinus Officinalis. Zhong Yao Cai 2008, 31, 877–879.
- Shabtay, A.; Sharabani, H.; Barvish, Z.; Kafka, M.; Amichay, D.; Levy, J.; Sharoni, Y.; Uskokovic, M.R.; Studzinski, G.P.; Danilenko, M. Synergistic Antileukemic Activity of Carnosic Acid-Rich Rosemary Extract and the 19-nor Gemini Vitamin D Analogue in a Mouse Model of Systemic Acute Myeloid Leukemia. Oncology 2008, 75, 203–214.
- Huang, M.T.; Ho, C.T.; Wang, Z.Y.; Ferraro, T.; Lou, Y.R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of Skin Tumorigenesis by Rosemary and Its Constituents Carnosol and Ursolic Acid. Cancer Res. 1994, 54, 701–708.
- Singletary, K.; MacDonald, C.; Wallig, M. Inhibition by Rosemary and Carnosol of 7,12-DimethylbenzAnthracene (DMBA)-Induced Rat Mammary Tumorigenesis and in vivo DMBA-DNA Adduct Formation. Cancer Lett. 1996, 104, 43–48.
- Li Pomi, F.; Borgia, F.; Custurone, P.; Vaccaro, M.; Pioggia, G.; Gangemi, S. Role of HMGB1 in Cutaneous Melanoma: State of the Art. Int. J. Mol. Sci. 2022, 23, 9327.
- Huang, S.-C.; Ho, C.-T.; Lin-Shiau, S.-Y.; Lin, J.-K. Carnosol Inhibits the Invasion of B16/F10 Mouse Melanoma Cells by Suppressing Metalloproteinase-9 through down-Regulating Nuclear Factor-Kappa B and c-Jun. Biochem. Pharm. 2005, 69, 221–232.
- Cattaneo, L.; Cicconi, R.; Mignogna, G.; Giorgi, A.; Mattei, M.; Graziani, G.; Ferracane, R.; Grosso, A.; Aducci, P.; Schininà, M.E.; et al. Anti-Proliferative Effect of Rosmarinus officinalis L. Extract on Human Melanoma A375 Cells. PLoS ONE 2015, 10, e0132439.
- Tong, L.; Wu, S. The Mechanisms of Carnosol in Chemoprevention of Ultraviolet B-Light-Induced Non-Melanoma Skin Cancer Formation. Sci. Rep. 2018, 8, 3574.
- Sancheti, G.; Goyal, P.K. Effect of Rosmarinus officinalis in Modulating 7,12-Dimethylbenz(a)Anthracene Induced Skin Tumorigenesis in Mice. Phytother. Res. 2006, 20, 981–986.
- Yasunaga, J. Viral, Genetic, and Immune Factors in the Oncogenesis of Adult T-Cell Leukemia/Lymphoma. Int. J. Hematol. 2023.
- Honda, M.; Yamada, Y.; Tomonaga, M.; Ichinose, H.; Kamihira, S. Correlation of Urinary 8-Hydroxy-2′-Deoxyguanosine (8-OHdG), a Biomarker of Oxidative DNA Damage, and Clinical Features of Hematological Disorders: A Pilot Study. Leuk Res. 2000, 24, 461–468.
- Hutchison, T.; Malu, A.; Yapindi, L.; Bergeson, R.; Peck, K.; Romeo, M.; Harrod, C.; Pope, J.; Smitherman, L.; Gwinn, W.; et al. The TP53-Induced Glycolysis and Apoptosis Regulator Mediates Cooperation between HTLV-1 P30II and the Retroviral Oncoproteins Tax and HBZ and Is Highly Expressed in an in vivo Xenograft Model of HTLV-1-Induced Lymphoma. Virology 2018, 520, 39–58.
- Ishida, Y.; Yamasaki, M.; Yukizaki, C.; Nishiyama, K.; Tsubouchi, H.; Okayama, A.; Kataoka, H. Carnosol, Rosemary Ingredient, Induces Apoptosis in Adult T-Cell Leukemia/Lymphoma Cells via Glutathione Depletion: Proteomic Approach Using Fluorescent Two-Dimensional Differential Gel Electrophoresis. Hum. Cell 2014, 27, 68–77.
- Visanji, J.M.; Thompson, D.G.; Padfield, P.J. Induction of G2/M Phase Cell Cycle Arrest by Carnosol and Carnosic Acid Is Associated with Alteration of Cyclin A and Cyclin B1 Levels. Cancer Lett. 2006, 237, 130–136.
More
