TPPP-like proteins contain one or more p25alpha domains. They obtained their name after tubulin polymerization promoting protein (TPPP1), the first identified member of this protein family. Originally, it was named p25alpha protein, which became the eponym of the domain. Myzozoans are a monophyletic clade, and a sister clade to the Ciliata, within Alveolata.
- apicortin
- TPPP
- Myzozoa
- chrompodellids
- dinoflagellates
1. Introduction
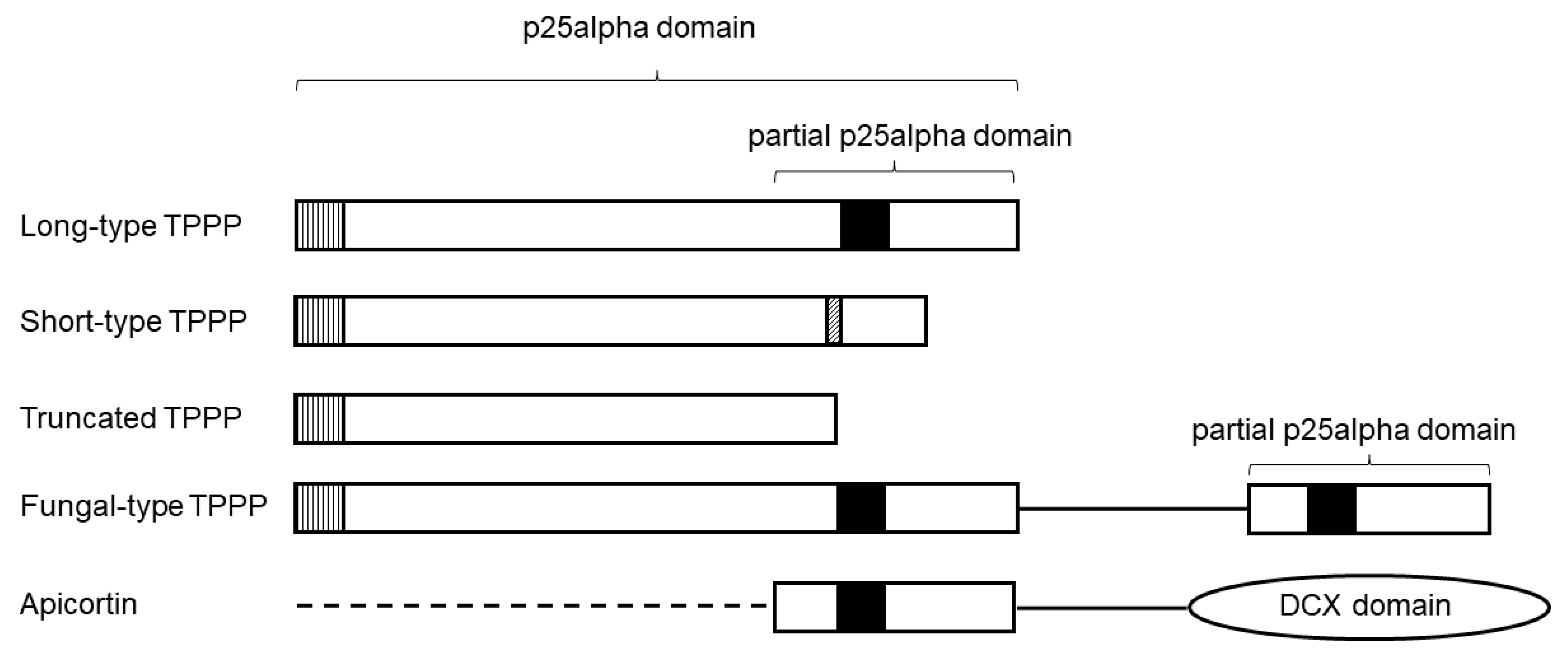
2. TPPP-like Proteins in Myzozoa
2.1. Apicortin
Apicortins unite two conserved domains, a DCX motif and a partial p25alpha sequence, that are separately found in other proteins, in doublecortins and TPPPs, respectively [7] (Figure 1). The DCX domain is named after the brain-specific X-linked gene doublecortin [28][17]. Both the p25alpha and the DCX domains play an important role in the stabilization of microtubules [2,3,28,29][2][3][17][18]; thus, a similar role was suggested for apicortin [7]. Apicortin was originally thought to occur only in apicomplexans and in the placozoan animal T. adhaerens [7]. Later, it was found that its occurrence is broader than thought earlier: it is present in chromerids (Chromera velia and Vitrella brassicaformis) [30][19] and in flagellated fungi [6,31,32][6][20][21]. The presence of apicortin in chromerids was not surprising, given the phylogenetic proximity and the structural similarity of chromerids and apicomplexans. BLASTP analyses [22] were performed on myzozoan protein and nucleotide sequences available at the NCBI webpage. Since apicortins contain two different domains, various domain databases were also checked for proteins with both the DCX and the partial p25alpha domains that BLAST may not have been able to detect. The results of the search, i.e., the new apicortins not known before, are listed in Table 1. They were found in all myzozoan phyla, in Apicomplexa, chrompodellids, dinoflagellates, and Perkinsozoa. The newly re-interpreted [36,37][23][24] squirmids [38][25] (Digyalum oweni [39][26]) also possess it (the list of apicortins identified earlier is published in the references [6,7,31][6][7][20]).| Species | Accesion Number (TSA) |
Class | Order | Identity with T. adhaerens Apicortin 3, % |
|---|---|---|---|---|
| Apicomplexans | ||||
2.3. Multidomain Proteins Containing Short p25alpha Domains
As expected, the BLAST search found no truncated-, long-, or fungal-type TPPP-like proteins in myzozoan species. These TPPP-like proteins are specific for Endopterygota, Opisthokonta, and fungi, respectively. However, several multidomain proteins have been identified that contain two short-type p25alpha domains in addition to others (Table 3).| Species | Accession Number (Protein) |
p25alpha Domains | Other Domains |
Length (aa) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Haemoproteus columbae | |||||||||
| GGWD01002623 | |||||||||
| Chrompodellids | |||||||||
| Aconoidasida | Haemosporida | 41.21 | |||||||
| Chromera velia | Cvel_4181 1,12 | 2 | 232 | Cardiosporidium cionae | KAF8822549 1 | Aconoidasida | Nephromycida | 36.67 | |
| Cvel_31116 1 | 2 | EF-hand | 499 | Nephromyces sp. ex Molgula occidentalis | GHIL01104982 | Aconoidasida | Nephromycida | 40.35 | |
| Vitrella brassicaformis | CEL98751 | 2 | EF-hand | 433 | Eleutheroschizon duboscqi | GHVT01063535 | Conoidasida | Protococcidiorida | 40.57 |
| Squirmids | Ancora sagittata | GHVO01023203 2 | Conoidasida | Eugregarinorida | 36.25 | ||||
| Cephaloidophora cf. communis | GHVH01004777 | Conoidasida | Eugregarinorida | 40.00 | |||||
| Lankesteria abbotti | HBHB01002866 2 | Conoidasida | Eugregarinorida | ||||||
| Digyalum oweni | GHRU01002363 2 | 2 | 399 | ||||||
| Dinoflagellates | 33.82 | ||||||||
| Polarella glacialis | CAE8635994 | 2 | EF-hand | 344 | Porospora cf. gigantea | KAH0481109 1 | Conoidasida | Eugregarinorida | 39.52 |
| CAE8640696 | 2 | Siedleckia nematoides | GHVV01274235 2 | Conoidasida | Eugregarinorida (Blastogregarinorida) |
51.35 | |||
| Selenidium pygospionis | GHVN01000425 2 | Conoidasida | Archigregarinorida | 35.66 | |||||
| Rhytidocystis sp. ex Travisia forbesii | GHVS01047420 2 GHVS01057697 2 |
Marosporida | Agamococcidiorida | 37.65 37.11 |
|||||
| Chrompodellids | |||||||||
| Alphamonas edax | GDKI01002741 | 36.26 | |||||||
| Colpodella angusta | GDKK01042800 | 40.88 | |||||||
| Squirmids. | |||||||||
| Digyalum oweni | GHRU01063100 | 48.52 | |||||||
| Dinoflagellates | |||||||||
| Karenia papilionacea | GJRB01069842 2 GFLM01035203 2 |
Dinophyceae | Gymnodiniales | 21.86 24.40 |
|||||
| Karlodinium armiger | GJRA01036780 GJQZ01074857 |
Dinophyceae | Gymnodiniales | 30.17 29.48 |
|||||
| Oxyrrhis marina | HBQX01034077 HBIT01006257 |
Dinophyceae | Oxyrrhinales | 33.33 33.33 |
|||||
| Symbiodinium sp. clade D | HBTB01100466 | Dinophyceae | Suessiales | 38.31 | |||||
| Perkinsids | |||||||||
| Perkinsus chesapeaki | KAF4672084 1 | Perkinsida | 23.90 | ||||||
| Perkinsus olseni | KAF4750811 1 | Perkinsida | 23.57 |
2.2. Short-Type TPPP
Short-type TPPPs have not been previously systematically investigated. They occur in various protists, mostly in Ciliata, Euglenozoa, and Chlorophyta [1], and they are present in Apicomlexa and Perkinsozoa [7]. Based on BLAST search, new short-type TPPPs were found in many myzozoans, not only in Apicomplexa and Perkinsozoa, but also in chrompodellids and dinoflagellates (Table 2). Within the apicomplexans, short-type TPPPs were found in Haemosporida, Piroplasmida, Eimeriidae, and Sarcocystidae, but they were absent in Cryptosporidiidae [7]. This analysis showed that they also occur in Nephromycida, gregarines (Eu-, Blasto-, and Archigregarinorida) and the recently defined class, Marosporida [36][23]. There are some characteristic sequences in short-type TPPPs. For example, one of the sequences, which is common with long-type TPPPs, is the L(V)xxxF(Y)xxF at the very beginning of the p25alpha domain. Another one is a GGP sequence in the C-terminal half of the protein (Figure 1). The length of the proteins varies between 120 and 170 amino acids.| Species | Accesion Number (TSA) |
Class | Order | Identity with T. thermophila TPPP 5, % |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Apicomplexans | |||||||||
| Haemoproteus columbae | GGWD01012446+ GGWD01012443 | Aconoidasida | Haemosporida | 48.61 | |||||
| Hepatocystis sp. ex Piliocolobus tephrosceles |
VWU48670 1 | Aconoidasida | Haemosporida | 42.07 | |||||
| Plasmodium gallinaceum | XP_028529806 1 | Aconoidasida | Haemosporida | 44.83 | |||||
| Cardiosporidium cionae | KAF8819752 1 | Aconoidasida | Nephromycida | 50.68 | |||||
| Nephromyces sp. ex Molgula occidentalis | GHIL01028850 | Aconoidasida | Nephromycida | 49.32 | |||||
| Babesia microti | XP_012649535 1 | Aconoidasida | Piroplasmida | 27.05 | |||||
| Cyclospora cayetanensis | XP_022592352 1 | Conoidasida | Eucoccidiorida | 56.55 | |||||
| Neospora caninum | XP_003880535 1 XP_003883867 1 |
Conoidasida | Eucoccidiorida | 56.94 50.34 |
|||||
| Eleutheroschizon duboscqi | GHVT01063834 | Conoidasida | Protococcidiorida; | 55.56 | |||||
| Ancora sagittata | GHVO01049457 | Conoidasida | Eugregarinorida | 3730 | |||||
| Cephaloidophora cf. communis | GHVH01010051 | Conoidasida | Eugregarinorida | 44.59 | |||||
| EF-hand | 347 | ||||||||
| CAE8623567 | 2 | EF-hand | 637 | Gregarina niphandrodes | GNI_040770 2 | Conoidasida | Eugregarinorida | 37.04 | |
| Polyrhabdina sp. | GHVP01031609 3 | Conoidasida | Eugregarinorida | 35.64 | |||||
| Porospora cf. gigantea | KAH0473834 KAH0475585 |
Conoidasida | Eugregarinorida | 34.01 36.05 |
|||||
| Siedleckia nematoides | GHVU01040477 GHVV01097121 |
Conoidasida | |||||||
| Eugregarinorida | (Blastogregarinorida) | 52.03 | 48.85 |
||||||
| 1368 | Selenidium pygospionis | GHVN01019431 4 | Conoidasida | Archigregarinorida | 47.30, 45.64 | ||||
| CAE7264691 | 2 | 333 | Rhytidocystis sp. ex Travisia forbesii | GHVS01062019 GHVS01062016 |
Marosporida | Agamococcidiorida | 41.22 43.24 |
||
| Chrompodellids | |||||||||
| Chromera velia | HBKZ01015069 HBKZ01021816 |
||||||||
| Symbiodinium sp. KB8 | CAE7914847 | sec 7; Ank_2, Nc, and others 10 | 2627 | ||||||
| CAE7947347 | 2 | PTZ00121 11 | 2004 | 61.38 | |||||
| Symbiodinium natans | 40.69 | ||||||||
| CAE7514938 | 2 | 338 | Vitrella brassicaformis | CEM02660 1 | 50.99 | ||||
| CAE7230103 | 2 | Nc | 735 | Alphamonas edax | GDKI01002338 | 56.16 | |||
| Symbiodinium microadriaticum | CAE7819146 | Nc | 1367 | Colpodella angusta | GDKK01046869 | 45.77 | |||
| Voromonas pontica | |||||||||
| GDKH01013421 3 | 57.69 | ||||||||
| OLP89249 | 2 | Nc | 1405 | Squirmids | |||||
| OLQ07037 | 2 | PTZ00121 | 2338 | ||||||
| CAE7562082 | 2 | PTZ00121 | 2161 | Digyalum oweni | GHRU01021184 | 45.27 | |||
| Symbiodinium necroappetens | CAE7814517 | 2 | sec 7; Nc | 1356 | Dinoflagellates | ||||
| CAE7554473 | 2 | PTZ00121 | 1082 | Karenia mikimotoi | GHKS01124154 | Dinophyceae | Gymnodiniales | ||
| Symbiodinium pilosum | CAE7666671 | 2 | Nc | 45.39 | |||||
| 1354 | Gambierdiscus australes | HBLT01006012 | Dinophyceae | Gonyaulacales | 53.24 | ||||
| Perkinsids | Dinophysis acuminata | GKBP01057714 | Dinophyceae | Dinophysiales | 30.07 | ||||
| 27.69 | |||||||||
| Symbiodinium natans | CAE7214312 1 | Dinophyceae | Suessiales | 28.69 | |||||
| Symbiodinium microadriaticum | CAE7469644 1 | Dinophyceae | Suessiales | 27.69 | |||||
| Perkinsids | |||||||||
| Perkinsus marinus | XP_002767104 1 | Perkinsida | 32.47 |
| Cladocopium goreaui | ||||
| CAI4012958 | ||||
| 2 | ||||
| Nc | ||||
| 3 | ||||
| and others | ||||
| 4 | ||||
| 1808 | ||||
| CAI4010985 | 2 | PRK08691 5 | 1704 | |
| CAI3986388 | 2 | several 6 | 1281 | |
| Symbiodinium sp. CCMP2592 | CAE7826117 | 2 | sec 7 7; Ank_2 8, Nc and others 9 | 2611 |
| CAE7226582 | 2 | 333 | ||
| Symbiodinium sp. CCMP2456 | CAE7634140 | 2 | sec 7; Nc | |
| Perkinsus chesapeaki | ||||
| KAF4658947 | ||||
| 2 | ||||
| 349 | ||||
| Symbiodinium sp. CCMP2456 | CAE7788281 1 | Dinophyceae | Suessiales | |
| Perkinsus olseni | KAF4688416 | 2 | 358 | |
| KAF4711908 | 2 | Ank_2 | 445 | |
| KAF4678978 | 2 | Ank_2 | 489 |
3. Possible Function of TPPP-like Proteins in Myzozoa
3.1. Tubulin-Based Structural Elements of Apicomplexa and Other Myzozoa
It is known that TPPP binds to tubulin, promotes its polymerization into microtubules, and stabilizes them [2,3,][2][3]. This function is conserved in animals from sponges to mammals [12]. Tubulin-based cytoskeletal elements of apicomplexans have distinct traits, which may be related to the unique biology of these parasites [48][37]. In principle, TPPP-like proteins can interact with and stabilize any of these microtubular elements, which has been confirmed experimentally.
Apicomplexans and their relatives possess discrete populations of microtubules and other tubulin polymers. Subpellicular microtubules and spindle microtubules are associated with the apical polar ring and with centrioles as the microtubule-organizing center, respectively. Axonemal microtubules are present in species possessing flagella in any of their life stages. Beside microtubules, some myzozoan species possess another polymer form of tubulin, the conoid fibers. They are similar to microtubules but their subunits are curled into an extremely tight coil, where tubulin is arranged into a polymer form that is different from typical microtubules [49]. Both the apical polar ring and the conoid are components of the namesake of apicomplexans, the apical complex, which was originally used for feeding in the common ancestor of apicomplexans and other myzozoans but was transformed for infection when the parasitism evolved [21,49].
Apicomplexans and their relatives possess discrete populations of microtubules and other tubulin polymers. Subpellicular microtubules and spindle microtubules are associated with the apical polar ring and with centrioles as the microtubule-organizing center, respectively. Axonemal microtubules are present in species possessing flagella in any of their life stages. Beside microtubules, some myzozoan species possess another polymer form of tubulin, the conoid fibers. They are similar to microtubules but their subunits are curled into an extremely tight coil, where tubulin is arranged into a polymer form that is different from typical microtubules [38]. Both the apical polar ring and the conoid are components of the namesake of apicomplexans, the apical complex, which was originally used for feeding in the common ancestor of apicomplexans and other myzozoans but was transformed for infection when the parasitism evolved [38][39].It should be noted that the apical complex is complete only in some apicomplexans (e.g., Eimeriidae, Sarcocystidae, Cryptosporidiidae, and gregarines) [49]; other species of this phylum (in Piroplasmida and Haemosporida) lost the conoid, but they retained an apical polar ring; moreover, a conoid-like structure was found in the ookinete stage of Plasmodium gallinaceum [51] and in another haemosporidian, Leucocytozoon simondi [52]. Chrompodellids, but not V. brassicaformis [53], and perkinsids have an uncomplete ’pseudoconoid’, while dinoflagellates lack it but instead, they have another specialized tubular structure called peduncle [54].
It should be noted that the apical complex is complete only in some apicomplexans (e.g., Eimeriidae, Sarcocystidae, Cryptosporidiidae, and gregarines) [38]; other species of this phylum (in Piroplasmida and Haemosporida) lost the conoid, but they retained an apical polar ring; moreover, a conoid-like structure was found in the ookinete stage of Plasmodium gallinaceum [40] and in another haemosporidian, Leucocytozoon simondi [41]. Chrompodellids, but not V. brassicaformis [42], and perkinsids have an uncomplete ’pseudoconoid’, while dinoflagellates lack it but instead, they have another specialized tubular structure called peduncle [43].3.2. Connection between Apical Complex/Conoid and Apicortin
The apical complex can be divided into three components, namely, the apical cap, the conoid, and the secretory organelles, micronemes, and rhoptries [49][38]. The conoid, which plays an important role in the host cell invasion, is best described from the apicomplexan families Eimeriidae and Sarcocystidae (Sarcocystis, Toxoplasma, Neospora, etc.). Apicortin seems to be in strict relation with this organelle. In T. gondii, apicortin was shown to be localized exclusively at the conoid and is essential for providing its correct structure and function [14,55][14][44]. Tubulin disappeared or was present in a reduced amount in the apical complex of an apicortin-null mutant, while microtubules were normal [55][44]. The deletion of apicortin resulted in shorter and misshaped conoids [55][44]. These defects were reversed by the expression of the apicortin coding sequence. Apicortin possesses two microtubule-binding domains (a partial p25alpha and a DCX), which stabilize and bundle microtubules [2,3,28,29][2][3][17][18]. Thus, apicortin is an ideal protein for stabilizing the tubulin-based conoid fibers. This stabilizing effect of apicortin was shown in a reconstituted in vitro system as well [14].
In P. falciparum, which has no conoid, apicortin is localized at the apical end and may be involved in the formation of the apical complex [56][45]. In both T. gondii and P. falciparum, the downregulation of apicortin leads to impaired host cell invasion [14,15][14][15]. Interestingly, in mammalian, but not in avian, Plasmodium parasites, the p25alpha domain is degenerated, i.e., some otherwise conservative amino acids are missing. At this moment, it is not clear yet whether the presence of a conoid-like structure in the ookinete stage of the avian parasite P. gallinaceum [51][40] is in connection with these sequence differences, or whether it happened accidentally, and a similar structure will also be found in mammalian parasites in the future. However, this altered sequence does not affect the ability of P. falciparum apicortin to bind to tubulin; in silico docking showed that the amino acids of the p25alpha domain are involved in the binding [56][45].
3.3. Flagella and Short-Type TPPP
TPPP-like proteins occur predominantly in species that are flagellated. The eukaryotic flagellum is a microtubule-based organelle; thus, the connection is not surprising. Although the correlation between the incidence of flagellum and the p25alpha domain is strong [5,6][5][6], there is only some evidence for the functional relationship between TPPP-like proteins and the flagellum. They are related to short-type TPPPs. The connection was first shown in Chlamydomonas reinhardtii, a biflagellate green alga [57][46]. Its TPPP ortholog, FAP265 protein, can be found in the flagella, and is indispensable in their formation, as proven by using FAP265 null mutants [57][46]. Myzozoan species are flagellated; however, apicomplexans are in a special position in this respect [58][47]. The flagella present in the common ancestor of the Myzozoa (and Alveolata) were lost during evolution. In many parasitic apicomplexans, flagella are only found in male microgametes with exceptions, such as Piroplasmida and the Cryptosporidium genus, where no flagellum occurs. Flagellum formation in apicomplexans does not require the so-called intraflagellar transport proteins, but all elements of the flagellar apparatus assemble directly within the cytoplasm in a process termed exflagellation [59][48].
There are examples for the flagellum-connected role of short-type TPPPs in Apicomplexa. In Plasmodium genus, P. falciparum short-type TPPP (PFL1770c; XP_001350760) was shown to be essential for gametocytogenesis, using piggyBac transposon-mediated insertional mutagenesis, but the mutant failed to form mature gametocytes [60][49]. Recently, it has also been found that the TPPP (Py05543; EAA17578) is necessary for male gametocyte exflagellation in Plasmodium yoelli [61][50]. Py05543− KO parasites, obtained by CRISPR/Cas9-mediated genome editing, were deficient in the exflagellation of male gametes by observing deficient exflagellation center formation [61][50].
In this respect, it is noteworthy that Cryptosporidium species, which lack flagella [62–64][51][52][53], do not have short-type TPPPs. This can be interpreted as meaning that the parasite does not need this protein. A similar loss was described in Cryptosporidium for δ- and ε-tubulin genes/proteins, which are marker proteins for the flagellum and basal bodies [50]. Piroplasmids also have no flagella, not even in microgametes [65][54]. Their short-type TPPPs are the shortest among all short-type TPPPs. The lengths of TPPP of Theileria annulata, B. microti, and B. bovis are 121, 123, and 124 amino acids, respectively. The length of most short-type TPPPs is approximately 140–150 amino acids. Furthermore, among all apicomplexan TPPPs, the piroplasmid TPPPs are the most divergent. With the loss of the flagellum, their function may have been lost, so they evolved faster, and perhaps acquired a new function.
3.4. Function of the p25alpha Domain-Containing Multidomain Proteins
WResearchers find very different proteins in this group. Their length varies between 232 and 2627 amino acids. Interestingly, there are two short-type p25alpha domains in all of them. Sometimes, they do not have another domain; this type of protein is not found in apicomplexans and colpodellids, but is present in chromerids, perkinsids, and dinoflagellates. There is such a protein also in D. oweni; this species was previously classified as an apicomplexan, but according to the latest phylogenetic classification, it belongs to a separate group, the squirmids, which are sisters to (apicomplexans and chrompodellids) [36,37][23][24]. The fact that D. oweni has this type of protein (two p25alpha domains and no other domains) fits into this picture; i.e., D. oweni is not an apicomplexan. The function of these proteins is unknown; it would be expected that, similar to short-type TPPPs and apicortins, they exert their effect by binding to a tubulin polymer.
Only in dinoflagellates can we find larger proteins consisting of 1–2 thousand amino acids that contain more than three domains, including catalytic ones. For these proteins, the additional domains determine the function, e.g., adenylate cyclase or methyltransferase activity. The role of the p25alpha domains is unknown; however, there are cases when the importance of the tubulin-binding ability is likely, e.g., in the alpha-tubulin suppressor domain-containing protein CAI3986388 (Cladocopium goreaui). In the narrow sense, I do not consider these proteins to be TPPP-like ones.
4. Evolutionary Considerations
Among the TPPP-like proteins, short-type TPPPs and apicortins occur in all myzozoan phyla. Both experimental results and phylogenetic considerations indicate that these tubulin-binding proteins play an important role in the structure and/or function of the flagellum and the apical complex/conoid, respectively. Moreover, an evolutionary connection between the flagellum and apical complex was proposed [49,50][38][55]. Recent results suggest that the conoid complex evolved from flagellar components [66,67][56][57] or the flagellar root apparatus [49,68][38][58]; that is, ‘the apical complex is the most conspicuously retained element of the associated flagellar root structures’ [49][38].
In light of this, it is not surprising that short TPPPs are also common in Ciliata, the sister phylum of Myzozoa, since their surface is covered by hundreds of cilia; they usually contain several paralogs of short-type TPPPs. However, they do not have apicortin, and they do not possess conoid or any similar structural elements. The short-type TPPP is also present in other protists (Euglenozoa and Chlorophyta), which are also flagellated. Myzozoans are flagellated, but in apicomplexans, only the male gametes have flagellum/flagella in some species. If not, then the loss of the flagellum resulted in either the loss of TPPPs (Cryptosporidium) or a degenerate protein significantly diverging from other TPPPs (Piroplasmida). The conoid is also absent from some apicomplexans (Piroplasmida and Haemosporida), but despite this, apicortin is present in almost all of them. There can be several reasons for this. On the one hand, conoid-like structures are also found in some of these species [51[40][41],52], and on the other hand, the apical polar ring is also present in these cases; furthermore, since these species once possessed a conoid, the apicortin remained as a relic. The only exception is B. microti, which has an extremely small genome [40][27] and lacks the ‘redundant’ protein.
WeIt cannot be ignored the fact that apicortin is also found in some Opisthokonta (flagellated fungi, the placozoan T. adhaerens) [6[6][59],69], which do not have a conoid or similar structure. These apicortins differ from apicomplexan orthologs in that, although they have a partial p25alpha domain and the entire DCX domain, the opisthokont apicortin lacks the long, disordered N-terminal region [6,[6][34],44], which appears to be necessary for the formation of the proper conoid structure [14,][14]. Their function is probably to stabilize tubulin polymers/microtubules in these species as well; this is also indicated by the fact that apicortin is the only TPPP-like protein in T. adhaerens and some flagellated fungi [6,7][6][7]. Since apicortin is present only in these few primitive opisthokonts apart from Myzozoa, it is possible that the opisthokonts acquired this protein from myzozoans by horizontal gene transfer. This is supported by the high degree of sequence identity and similarity between apicomplexan and chromerid apicortins on the one hand, and Trichoplax and fungal apicortins on the other, which can reach 53% and 67%, respectively, which is roughly the same value as the similarity of apicomplexan apicortins to each other. The similarity exists not only between the domain sequences, but also in the interdomain linker. Since the last common ancestor of myzozoans and opisthokonts could be close to the first eukaryote, it is extremely unlikely that the gene remained so conserved for that much time, and horizontal gene transfer is the best explanation.
5. Conclusions
A common feature of TPPP-like proteins is that they contain one or more p25alpha domains [1]. They occur only in eukaryotes, and like their name indicates, they are tubulin-binding proteins [2,3,][2][3]. This trait is conserved in animals, as it is present in everything from sponges to humans [13]. The phylogenetic distribution of TPPP-like proteins is widespread in protists as well, but not in plants [1]. The role of TPPP-like proteins may be quite different in protists than in humans. Short-type TPPPs and apicortins are found in Myzozoa, a major monophyletic group of Alveolata. Although there are few experimental examples so far, short-type TPPPs have a role in the flagellar structures, interacting with tubulin polymers, and perhaps play a role in the exflagellation of microgametes [60,61][49][50]. Apicortin may play a crucial role in the formation of the conoid/apical complex [14,15,55,56][14][15][44][45]. Thus, TPPP-like proteins may be potential therapeutic targets for the treatment of diseases such as malaria and toxoplasmosis [50][55].
References
- Orosz, F. A new protein superfamily: TPPP-like proteins. PLoS ONE 2012, 7, e49276.
- Hlavanda, E.; Kovács, J.; Oláh, J.; Orosz, F.; Medzihradszky, K.F.; Ovádi, J. Brainspecific p25 protein binds to tubulin and microtubules and induces aberrant microtubule assemblies at substoichiometric concentrations. Biochemistry 2002, 417, 8657–8664.
- Tirián, L.; Hlavanda, E.; Oláh, J.; Horváth, I.; Orosz, F.; Szabó, B.; Kovács, J.; Szabad, J.; Ovádi, J. TPPP/p25 promotes tubulin assemblies and blocks mitotic spindle formation. Proc. Natl. Acad. Sci. USA 2003, 100, 13976–13981.
- Takahashi, M.; Tomizawa, K.; Ishiguro, K.; Sato, K.; Omori, A.; Sato, S.; Shiratsuchi, A.; Uchida, T.; Imahori, K. A novel brainspecific 25 kDa protein (p25) is phosphorylated by a Ser/Thr-Pro kinase (TPK II) from tau protein kinase fractions. FEBS Lett. 1991, 289, 37–43.
- Orosz, F.; Ovádi, J. TPPP orthologs are ciliary proteins. FEBS Lett. 2008, 582, 3757–3764.
- Orosz, F. On the TPPP-like proteins of flagellated Fungi. Fungal Biol. 2021, 125, 357–367.
- Orosz, F. Apicortin, a unique protein, with a putative cytoskeletal role, shared only by apicomplexan parasites and the placozoan Trichoplax adhaerens. Infect. Genet. Evol. 2009, 9, 1275–1286.
- Lehotzky, A.; Tirián, L.; Tökési, N.; Lénárt, P.; Szabó, B.; Kovács, J.; Ovádi, J. Dynamic targeting of microtubules by TPPP/p25 affects cell survival. J. Cell Sci. 2004, 117, 6249–6259.
- Lehotzky, A.; Lau, P.; Tokési, N.; Muja, N.; Hudson, L.D.; Ovádi, J. Tubulin polymerization-promoting protein (TPPP/p25) is critical for oligodendrocyte differentiation. Glia 2010, 58, 157–168.
- Kovács, G.G.; László, L.; Kovács, J.; Jensen, P.H.; Lindersson, E.; Botond, G.; Molnár, T.; Perczel, A.; Hudecz, F.; Mezo, G.; et al. Natively unfolded tubulin polymerization promoting protein TPPP/p25 is a common marker of alpha-synucleinopathies. Neurobiol. Dis. 2004, 17, 155–162.
- Orosz, F.; Kovács, G.G.; Lehotzky, A.; Oláh, J.; Vincze, O.; Ovádi, J. TPPP/p25: From unfolded protein to misfolding disease: Prediction and experiments. Biol. Cell 2004, 96, 701–711.
- Ferreira, N.; Gram, H.; Sorrentino, Z.A.; Gregersen, E.; Schmidt, S.I.; Reimer, L.; Betzer, C.; Perez-Gozalbo, C.; Beltoja, M.; Nagaraj, M.; et al. Multiple system atrophy-associated oligodendroglial protein p25α stimulates formation of novel α-synuclein strain with enhanced neurodegenerative potential. Acta Neuropathol. 2021, 142, 87–115.
- Oláh, J.; Szénási, T.; Szabó, A.; Kovács, K.; Lőw, P.; Štifanić, M.; Orosz, F. Tubulin binding and polymerization promoting properties of Tubulin Polymerization Promoting Proteins are evolutionarily conserved. Biochemistry 2017, 56, 1017–1024.
- Leung, J.M.; Nagayasu, E.; Hwang, Y.C.; Liu, J.; Pierce, P.G.; Phan, I.Q.; Prentice, R.A.; Murray, J.M.; Hu, K. A doublecortin-domain protein of Toxoplasma and its orthologues bind to and modify the structure and organization of tubulin polymers. BMC Mol. Cell Biol. 2020, 21, 8.
- Chakrabarti, M.; Joshi, M.; Kumari, G.; Singh, P.; Shoaib, R.; Munjal, A.; Kumar, V.; Behl, A.; Abid, M.; Garg, S.; et al. Interaction of Plasmodium falciparum apicortin with ?- and ?-tubulin is critical for parasite growth and survival. Sci. Rep. 2021, 11, 4688.
- Orosz, F. Truncated TPPP—An Endopterygota-specific protein. Heliyon 2021, 7, e07135.
- Sapir, T.; Horesh, D.; Caspi, M.; Atlas, R.; Burgess, H.A.; Wolf, S.G.; Francis, F.; Chelly, J.; Elbaum, M.; Pietrokovski, S.; et al. Doublecortin mutations cluster in evolutionarily conserved functional domains. Hum. Mol. Genet. 2000, 9, 703–712.
- Kim, M.H.; Cierpicki, T.; Derewenda, U.; Krowarsch, D.; Feng, Y.; Devedjiev, Y.; Dauter, Z.; Walsh, C.A.; Otlewski, J.; Bushweller, J.H.; et al. The DCX domain tandems of doublecortin and doublecortin-like kinase. Nat. Struct. Biol. 2003, 10, 324–333.
- Orosz, F. On the TPPP protein of the enigmatic fungus, Olpidium—Correlation between the incidence of p25alpha domain and that of the eukaryotic flagellum. Int. J. Mol. Sci. 2022, 23, 13927.
- Orosz, F. Wider than thought phylogenetic occurrence of apicortin, a characteristic protein of apicomplexan parasites. J. Mol. Evol. 2016, 82, 303–314.
- Orosz, F. Tubulin Polymerization Promoting Proteins (TPPPs) of Aphelidiomycota: Correlation between the incidence of p25alpha domain and the eukaryotic flagellum. J. Fungi 2023, 9, 376.
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402.
- Mathur, V.; Kwong, W.K.; Husnik, F.; Irwin, N.A.T.; Kristmundsson, Á.; Gestal, C.; Freeman, M.; Keeling, P.J. Phylogenomics identifies a new major subgroup of apicomplexans, Marosporida class nov., with extreme apicoplast genome reduction. Genome Biol. Evol. 2021, 13, evaa244.
- Mathur, V.; Na, I.; Kwong, W.K.; Kolisko, M.; Keeling, P.J. Reconstruction of plastid proteomes of apicomplexans and close relatives reveals the major evolutionary outcomes of cryptic plastids. Mol. Biol. Evol. 2023, 40, msad002.
- Cavalier-Smith, T. Gregarine site-heterogeneous 18S rDNA trees, revision of gregarine higher classification, and the evolutionary diversification of Sporozoa. Eur. J. Protistol. 2014, 50, 472–495.
- Koura, E.A.; Grahame, J.; Owen, R.W.; Kamel, E.G. Digyalum oweni, gen. nov., sp. nov., a new and unusual gregarin protozoan from the gut of mollusc Littorina obtusata (Prosobranchia: Gastropoda). J. Egypt. Soc. Parasitol. 1990, 20, 53–59.
- Cornillot, E.; Hadj-Kaddour, K.; Dassouli, A.; Noel, B.; Ranwez, V.; Vacherie, B.; Augagneur, Y.; Brès, V.; Duclos, A.; Randazzo, S.; et al. Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res. 2012, 40, 9102–9114.
- Janouškovec, J.; Paskerova, G.G.; Miroliubova, T.S.; Mikhailov, K.V.; Birley, T.; Aleoshin, V.V.; Simdyanov, T.G. Apicomplexan-like parasites are polyphyletic and widely but selectively dependent on cryptic plastid organelles. Elife 2019, 8, e49662.
- Boisard, J.; Duvernois-Berthet, E.; Duval, L.; Schrével, J.; Guillou, L.; Labat, A.; Le Panse, S.; Prensier, G.; Ponger, L.; Florent, I. Marine gregarine genomes reveal the breadth of apicomplexan diversity with a partially conserved glideosome machinery. BMC Genom. 2022, 23, 485.
- Hunter, E.S.; Paight, C.; Lane, C.E. Metabolic contributions of an alphaproteobacterial endosymbiont in the apicomplexan Cardiosporidium ciona. Front. Microbiol. 2020, 11, 580719.
- Muñoz-Gómez, S.A.; Durnin, K.; Eme, L.; Paight, C.; Lane, C.E.; Saffo, M.B.; Slamovits, C.H. Nephromyces represents a diverse and novel lineage of the Apicomplexa that has retained apicoplasts. Genome Biol. Evol. 2019, 11, 2727–2740.
- Gile, G.H.; Slamovits, C.H. Transcriptomic analysis reveals evidence for a cryptic plastid in the colpodellid Voromonas pontica a close relative of chromerids and apicomplexan parasites. PLoS ONE 2014, 9, e96258.
- Janouškovec, J.; Tikhonenkov, D.V.; Burki, F.; Howe, A.T.; Kolísko, M.; Mylnikov, A.P.; Keeling, P.J. Factors mediating plastid dependency and the origins of parasitism in apicomplexans and their close relatives. Proc. Natl. Acad. Sci. USA 2015, 112, 10200–102007.
- Orosz, F. Apicomplexan apicortins possess a long disordered N-terminal extension. Infect. Genet. Evol. 2011, 11, 1037–1044.
- Bogema, D.R.; Yam, J.; Micallef, M.L.; Gholipourkanani, H.; Go, J.; Jenkins, C.; Dang, C. Draft genomes of Perkinsus olseni and Perkinsus chesapeaki reveal polyploidy and regional differences in heterozygosity. Genomics 2021, 113, 677–688.
- Warrenfeltz, S.; Kissinger, J.C.; EuPathDB Team. Accessing Cryptosporidium omic and isolate data via CryptoDB.org. Methods Mol. Biol. 2020, 2052, 139–192.
- Morrissette, N.S.; Sibley, L.D. Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. Rev. 2002, 66, 21–38.
- Dos Santos Pacheco, N.; Tosetti, N.; Koreny, L.; Waller, R.F.; Soldati-Favre, D. Evolution, composition, assembly, and function of the conoid in Apicomplexa. Trends Parasitol. 2020, 36, 688–704.
- Wiser, M.F. Unique Endomembrane Systems and Virulence in Pathogenic Protozoa. Life 2021, 11, 822.
- Patra, K.P.; Vinetz, J.M. New ultrastructural analysis of the invasive apparatus of the Plasmodium ookinete. Am. J. Trop. Med. Hyg. 2012, 87, 412–417.
- Brockley Paterson, W.; Desser, S.S. The polar ring complex in ookinetes of Leucocytozoon simondi (Apicomplexa: Haemosporida) and evidence for a conoid in haemosporidian ookinetes. Eur. J. Protistol. 1989, 24, 244–251.
- Füssy, Z.; Petra Masařová, P.; Kručinská, J.; Esson, H.J.; Oborník, M. Budding of the alveolate alga Vitrella brassicaformis resembles sexual and asexual processes in apicomplexan parasite. Protist 2017, 168, 80–91.
- Hansen, P.J.; Calado, A.J. Phagotrophic mechanisms and prey selection in free-living dinoflagellates. J. Eukaryot. Microbiol. 1999, 46, 382–389.
- Nagayasu, E.; Hwang, Y.C.; Liu, J.; Murray, J.M.; Hu, K. Loss of a doublecortin (DCX)-domain protein causes structural defects in a tubulin-based organelle of Toxoplasma gondii and impairs host-cell invasion. Mol. Biol. Cell 2017, 28, 411–428.
- Chakrabarti, M.; Garg, S.; Rajagopal, A.; Pati, S.; Singh, S. Targeted repression of Plasmodium apicortin by host microRNA impairs malaria parasite growth and invasion. Dis. Model. Mech. 2020, 13, dmm042820.
- Tammana, D.; Tammana, T.V.S. Chlamydomonas FAP265 is a tubulin polymerization promoting protein, essential for flagellar reassembly and hatching of daughter cells from the sporangium. PLoS ONE 2017, 12, e0185108.
- Orosz, F. Apicortin, a constituent of apicomplexan conoid/apical complex and its tentative role in pathogen-host interaction. Trop. Med. Infect. Dis. 2021, 6, 118.
- Avidor-Reiss, T.; Leroux, M.R. Shared and distinct mechanisms of compartmentalized and cytosolic ciliogenesis. Curr. Biol. 2015, 25, R1143–R1150.
- Ikadai, H.; Shaw Saliba, K.; Kanzok, S.M.; McLean, K.J.; Tanaka, T.Q.; Cao, J.; Williamson, K.C.; Jacobs-Lorena, M. Transposon mutagenesis identifies genes essential for Plasmodium falciparum gametocytogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, E1676–E1684.
- Zhang, C.; Li, D.; Meng, Z.; Zhou, J.; Min, Z.; Deng, S.; Shen, J.; Liu, M. Pyp25α is required for male gametocyte exflagellation. Pathog. Dis. 2022, 80, ftac043.
- Ostrovska, K.; Paperna, I. Cryptosporidium sp. of the starred lizard Agame stellio: Ultrastructure and life cycle. Parasitol. Res. 1990, 76, 712–720.
- Beĭer, T.V.; Sidorenko, N.V. An electron microscopic study of Cryptosporidium. II. The stages of gametogenesis and sporogony in Cryptosporidium parvum. Tsitologiia 1990, 32, 592–598.
- Tandel, J.; English, E.D.; Sateriale, A.; Gullicksrud, J.A.; Beiting, D.P.; Sullivan, M.C.; Pinkston, B.; Striepen, B. Life cycle progression and sexual development of the apicomplexan parasite Cryptosporidium parvum. Nat. Microbiol. 2019, 4, 2226–2236.
- Adl, S.M.; Simpson, A.G.; Farmer, M.A.; Andersen, R.A.; Anderson, O.R.; Barta, J.R.; Bowser, S.S.; Brugerolle, G.; Fensome, R.A.; Fredericq, S.; et al. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 2005, 52, 399–451.
- Morrissette, N.S.; Abbaali, I.; Ramakrishnan, C.; Hehl, A.B. The tubulin superfamily in apicomplexan parasites. Microorganisms 2023, 11, 706.
- De Leon, J.C.; Scheumann, N.; Beatty, W.; Beck, J.R.; Tran, J.Q.; Yau, C.; Bradley, P.J.; Gull, K.; Wickstead, B.; Morrissette, N.S. A SAS-6-like protein suggests that the Toxoplasma conoid complex evolved from flagellar components. Eukaryot. Cell 2013, 12, 1009–1019.
- Portman, N.; Šlapeta, J. The flagellar contribution to the apical complex: A new tool for the eukaryotic Swiss Army knife? Trends Parasitol. 2014, 30, 58–64.
- Lévêque, M.F.; Berry, L.; Besteiro, S. An evolutionarily conserved SSNA1/DIP13 homologue is a component of both basal and apical complexes of Toxoplasma gondii. Sci. Rep. 2016, 6, 27809.
- Ringrose, J.H.; van den Toorn, H.W.P.; Eitel, M.; Post, H.; Neerincx, P.; Schierwater, B.; Altelaar, A.F.M.; Heck, A.J.R. Deep proteome profiling of Trichoplax adhaerens reveals remarkable features at the origin of metazoan multicellularity. Nat. Commun. 2013, 4, 1408.
