You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Francesco Carlomagno.
Recombinant follicle-stimulating hormone (FSH) is commonly used for the treatment of female and male infertility FSH is composed of an α subunit, shared with other hormones, and a β subunit, which confers specificity of biological action by interacting with its surface receptor (FSHR), predominantly located in granulosa and Sertoli cells. Beyond the well-known effects of FSH on reproductive functions the attention has recently focused on the extra-gonadal effects of FSH, specifically on bone and adipose tissue metabolsm, the cardiovascular and immune systems and the prostate gland. FSH could therefore be involved in several pathological and physiological processes, which are still not completely understood.
- FSH
- extra-gonadal
- bone
- cardiovascular system
- immune system
- metabolism
- infertility
- prostate cancer
1. Introduction
Follicle-stimulating hormone (FSH) was first identified in 1930. This hormone plays a central role in mammals’ reproduction, and its synthesis is regulated by gonadotropin-releasing hormone (GnRH) pulsatile release, leading to the activation of the hypothalamic–pituitary–gonadal (HPG) axis, first during mini-puberty (lasting, in boys, from the first to the sixth/ninth month of life), and then in puberty [1].
FSH is a heterodimeric glycoprotein hormone made of two subunits (α and β), with a molecular weight of 28–30 kDa, secreted by the anterior pituitary gland. Its structure is shared with other glycoproteic hormones, including luteinizing hormone (LH), thyroid-stimulating hormone (TSH) and human chorionic gonadotropin (hCG). The common α subunit is coupled with the hormone-specific β subunits, and the presence of both is required for biological activity. The FSH β subunit consists of 111 amino acids, conferring specific biologic action and responsible for the interaction with the FSH receptor (FSHR) [2], localized on the surface of target cells, e.g., on the testes and ovaries (granulosa and Sertoli cells). The FSHR belongs to the family of G protein-coupled receptors, (GPCRs) involved in various signaling pathways (i.e., cAMP/PKA, PKC/MAPK, and Ca+/CaMKII) [3,4,5][3][4][5].
FSH plays a pivotal role in reproduction, stimulating antrum formation in secondary follicles, as well as growth and maturation in antral follicles in females, whereas in males FSH is responsible for testicular development and maintenance. In mature gonads, it acts on Sertoli cells, spermatogonia, primary and secondary spermatocytes, and drives spermatogenesis [6].
FSH preparations (urinary, purified, and recombinant) are widely used in the treatment of female infertility, but increasingly it is being used to treat males with hypogonadotropic hypogonadism or idiopathic infertility with inappropriately normal FSH levels.
According to the 2018 European Academy of Andrology (EAA) guidelines on oligo-astheno-teratozoospermia (OAT), FSH treatment can be offered to selected men who are normo-gonadotropic with idiopathic OAT, although with low evidence [7]. The Italian Society of Andrology and Sexual Medicine (SIAMS) recently suggested the use of FSH preparations to increase sperm concentration and motility in infertile normo-gonadotropic men with idiopathic OAT, with moderate evidence grading [8], although this is limited by the relatively high costs and off-label usage in some countries [9]. In hypogonadotropic hypogonadism the use of FSH is more easily recommended [10]. Beyond the well-known effects of FSH on male and female reproductive functions, and precisely because of its increasing usage in the treatment of infertility, the attention has recently focused on the extra-gonadal effects of FSH, along with the possible underlying mechanisms. Recent studies, indeed, have found that FSH receptors also exist in extra-gonadal tissues, such as immune cells (monocytes/macrophages and others) [11] and the vascular endothelium [12]. FSH could, therefore, be involved in other pathological and physiological processes, which are still not completely understood.
2. Gonadal Effects of FSH
In males, FSH activates Sertoli cell proliferation, first during fetal development, then during mini-puberty, and continuing at puberty, when it induces spermatogenesis, whilst LH induces Leydig cells’ steroidogenesis to produce androgens [13]. In adult life, FSH acts through its receptors on Sertoli cells and germ cells up to the secondary spermatocyte stage, promoting meiosis entry and limiting overall germ cell apoptosis. Testosterone (T) is responsible for the later spermatogenesis stages, comprising spermiogenesis [14]. In this regard, FSH and T independently regulate spermatogenesis in an additive, as well as a synergistic, manner [15], with strong crosstalk with the somatotropic axis [16]: T activates the androgen receptor (AR) in Sertoli cells to initiate the functional responses required for spermatogenesis [17], whereas FSH stimulates Sertoli cells to produce signaling molecules and nutrients to support sperm maturation [3,18][3][18]. Moreover, FSH is able to induce LH receptor expression on Leydig cells, therefore influencing steroidogenesis as well [19].3. Extra-Gonadal Effects of FSH
There are currently few studies in the male literature that evaluate the extragonadal effects of FSH. On the other hand, there is increasing interest in considering hormones’ noncanonical effects on different organs and tissues [20]. A lot of evidence comes from animal models and in vitro studies on blood from donors. Existing human clinical trials have been performed primarily in the setting of hypogonadism. An ideal clinical model is represented by patients with previous prostate cancer who underwent androgen deprivation therapy (ADT). Commonly ADT makes use of the following three therapeutic possibilities: (1) GnRH-antagonist (which causes a rapid decrease of FSH levels); (2) GnRH-agonist (responsible for an initial increase of FSH followed by a gradual decrease; (3) bilateral orchiectomy (which leads to very high FSH levels). A limited number of papers have compared the cortices of patients with hypogonadotropic and hypergonadotropic hypogonadism. Overall, results from in vivo clinical trials in the setting of hypogonadism should be interpreted with caution due to possible confounding of testosterone deficiency or (in the case of hypergonadotropic hypogonadism) of coexisting high LH levels. Finally, a very limited number of randomized control trials (RCTs) have analyzed the extragonadal effects of FSH as a primary outcome after FSH treatment for male infertility. A summary of the effects is provided in Figure 1.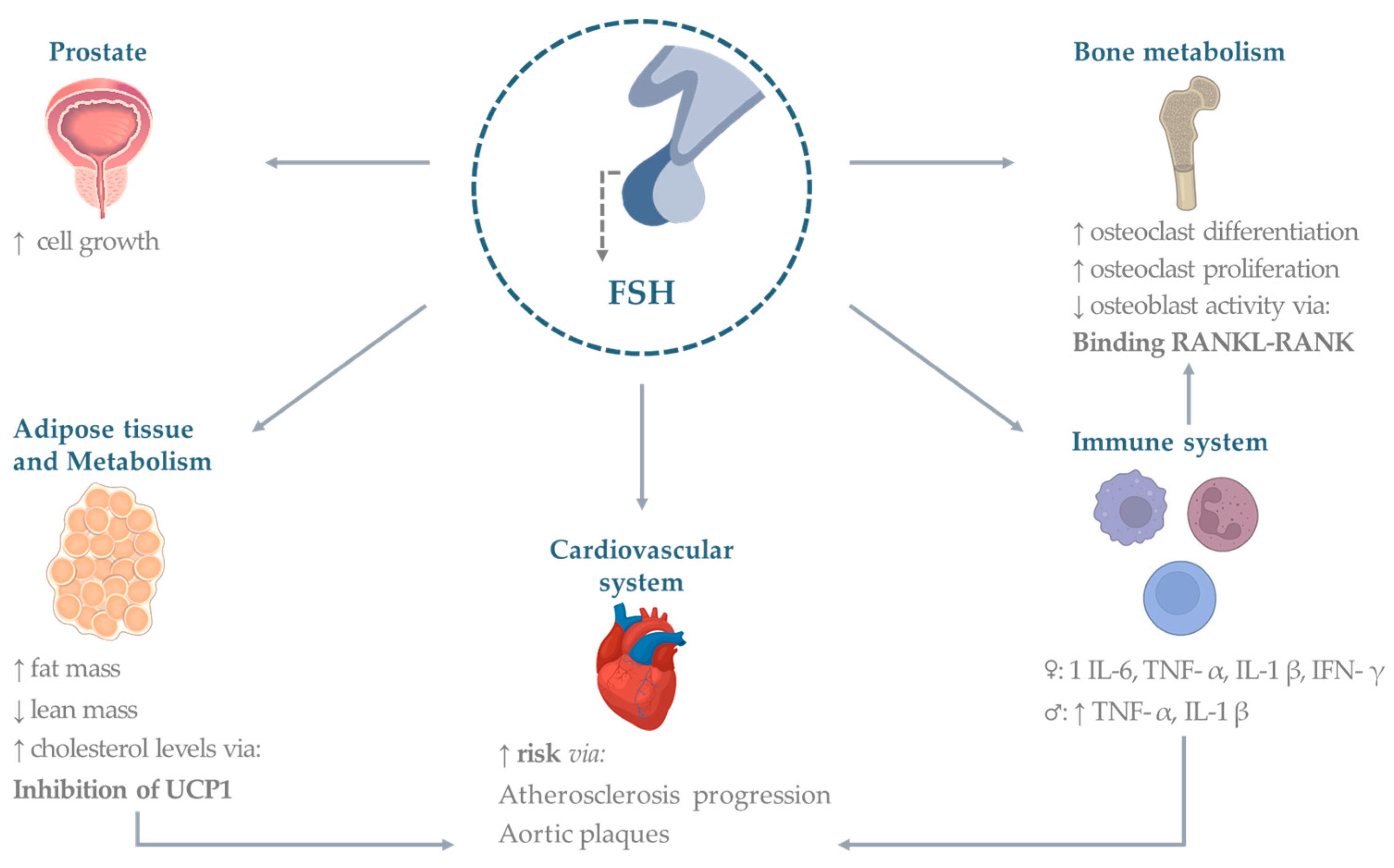
Figure 1. Extragonadal effects of FSH. Established and potential FSH effects on organs and systems. Abbreviations: TNF (tumor necrosis factor), IL (interleukin), UCP (uncoupling protein), IFN (interferon), RANK (Receptor activator of nuclear factor κ B), RANKL (Receptor activator of nuclear factor kappa-Β ligand), FSH (follicle-stimulating hormone). Figures made under Creative Commons License by using the Adobe Stock platform.
3.1. Bone
Estradiol is known to be the main hormone influencing bone remodeling. Therefore, osteoporosis is conceptually associated with a reduction of estrogens’ action [21]. However, FSH correlates with diminished bone mineral density even years before a decline in estradiol [21,22,23][21][22][23]. Data are still scarce; however, recent in vitro and preclinical studies have shown that FSH stimulates bone resorption by binding to its specific receptors on osteoclasts [24].
3.2. Cardiovascular System
Consolidated studies have shown how cardiovascular (CV) risk is associated with low estrogen levels in premenopausal women [45][25]. More recently, a direct role of FSH on CV risk has been proposed. The Study of Women’s Health Across the Nation (SWAN) correlated FSH levels’ trajectories with atherosclerosis development [46][26], finding that women experiencing low FSH rise after menopause may be at lower risk of atherosclerosis than those who experience either a medium or a high rise FSH over the transition. Munir et al., in the Assessment of the Transition of Hormonal Evaluation with Non-invasive imaging of Atherosclerosis (ATHENA-CT) study, showed how FSH was directly associated with the number of aortic plaques, using coronary CT angiography and carotid ultrasound [47][27]. Only a few studies investigated FSH-mediated effects in males. Animal models receiving androgen deprivation therapy (ADT) showed that the largest effects on metabolism, in terms of adiposity and glucose tolerance, and atherosclerosis, were induced, respectively, by orchiectomy, followed by GnRH-agonists and -antagonists, underlying a possible association between FSH levels and the severity of the phenotype [48][28].3.3. Adipose Tissue
A growing body of evidence demonstrates a possible role of FSH in regulating lipid metabolism, visceral adiposity, metabolic syndrome, and related diseases [54,55][29][30]. FSHRs have been shown to be expressed in adipocytes [56][31]. FSH acts by upregulating FSHR in adipocytes, which promotes increased fat accumulation [56,57][31][32]. Testing the effects of a polyclonal FSH antibody on bone mass, Liu et al. observed a sharp reduction of adipose tissue in treated wild type mice [58][33]. Moreover, FSH is able to induce the “beiging” of white adipocytes, contributing to the uncoupling mechanism of thermogenesis (with increased UCP1-rich beige-like adipose tissue) and leanness. Similar results were obtained in haploinsufficient (FSHR+/−) mice [58][33]. FSH also increases adipocyte volume, playing a role in visceral redistribution of body fat [57,58][32][33].3.4. Metabolism
Following the evidence of a higher prevalence of hypercholesterolemia in 400 postmenopausal women with high FSH levels [55][30], extragonadal FSH signaling was also investigated in relation to its supposed effects on lipid biosynthesis. An epidemiological investigation compared 153 pre-menopausal women to 124 peri-menopausal women and reported a direct, estradiol-independent correlation between FSH and cholesterol levels. Moreover, FSH administration in a peri-menopause mouse model resulted in increased cholesterol levels, and the effect could be reverted by blocking FSH signaling with an anti-FSHβ antibody or by FSHR gene ablation, indicating a direct and independent influence of FSH on hepatic cholesterol synthesis [63][34].3.5. Immune System
FSH appears to play a role in immune function and response. As previously reported, FSHR is expressed on immune cells, particularly in monocytes [24]. It was, therefore, hypothesized that FSH could influence the inflammatory response through the production of some cytokines. Data are reported for interferon-γ (IFN-γ), IL-1β [31,72,73][35][36][37] and IL-6 secreted from lipopolysaccharide (LPS) cultured monocytes [74][38]. A study on mice also described a role of FSH in stimulating IL-6 production from Sertoli cells [75][39]. According to some studies on CD4+ T cell cultures, isolated from peripheral blood mononuclear cells (PBMCs) of healthy donors, stimulation with FSH does not have pro-inflammatory effects [76,77][40][41].3.6. Prostate and Other Cancers
Increasing evidence has linked FSH in the development and progression of prostate cancer, as well as in the development of castration-resistant prostate cancer. Indeed, preclinical studies in chemically castrated mice suggest that FSH exerts a direct role on prostate cell growth [92][42]. Moreover, the presence of FSH, LH and their respective receptors has been reported in prostate cancer cells [93,94][43][44]. FSHR expression was found to be low-to-undetectable in normal prostate tissue and in benign prostatic hyperplasia, and consistently high in prostate cancer tissue [95][45]. Furthermore, FSHR may be relevant in prostate cancer progression, given its dense expression at the periphery of tumors [95][45]. One small clinical trial investigated the use of abarelix, a GnRH-antagonist, in prostate cancer patients who developed castration-resistant disease following orchiectomy, allowing a reduction in FSH (<5 mIU/L), and the results supported the hypothesis that depleting FSH may have a therapeutic role in castration-resistant prostate cancers. These data demonstrate that GnRH antagonists may have antineoplastic activity beyond their testosterone-reducing properties [96,97][46][47].References
- Spaziani, M.; Tarantino, C.; Tahani, N.; Gianfrilli, D.; Sbardella, E.; Lenzi, A.; Radicioni, A.F. Hypothalamo-Pituitary axis and puberty. Mol. Cell. Endocrinol. 2021, 520, 111094.
- Bhartiya, D.; Patel, H. An overview of FSH-FSHR biology and explaining the existing conundrums. J. Ovarian Res. 2021, 14, 144.
- Recchia, K.; Jorge, A.S.; Pessoa, L.V.F.; Botigelli, R.C.; Zugaib, V.C.; de Souza, A.F.; Martins, D.D.S.; Ambrosio, C.E.; Bressan, F.F.; Pieri, N.C.G. Actions and Roles of FSH in Germinative Cells. Int. J. Mol. Sci. 2021, 22, 10110.
- Simoni, M.; Gromoll, J.; Nieschlag, E. The follicle-stimulating hormone receptor: Biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 1997, 18, 739–773.
- Bonfil, D.; Chuderland, D.; Kraus, S.; Shahbazian, D.; Friedberg, I.; Seger, R.; Naor, Z. Extracellular signal-regulated kinase, Jun N-terminal kinase, p38, and c-Src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone beta-subunit promoter. Endocrinology 2004, 145, 2228–2244.
- Rannikko, A.; Penttila, T.L.; Zhang, F.P.; Toppari, J.; Parvinen, M.; Huhtaniemi, I. Stage-specific expression of the FSH receptor gene in the prepubertal and adult rat seminiferous epithelium. J. Endocrinol. 1996, 151, 29–35.
- Colpi, G.M.; Francavilla, S.; Haidl, G.; Link, K.; Behre, H.M.; Goulis, D.G.; Krausz, C.; Giwercman, A. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology 2018, 6, 513–524.
- Barbonetti, A.; Calogero, A.E.; Balercia, G.; Garolla, A.; Krausz, C.; La Vignera, S.; Lombardo, F.; Jannini, E.A.; Maggi, M.; Lenzi, A.; et al. The use of follicle stimulating hormone (FSH) for the treatment of the infertile man: Position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Investig. 2018, 41, 1107–1122.
- Chehab, M.; Madala, A.; Trussell, J.C. On-label and off-label drugs used in the treatment of male infertility. Fertil. Steril. 2015, 103, 595–604.
- Casarini, L.; Crepieux, P.; Reiter, E.; Lazzaretti, C.; Paradiso, E.; Rochira, V.; Brigante, G.; Santi, D.; Simoni, M. FSH for the Treatment of Male Infertility. Int. J. Mol. Sci. 2020, 21, 2270.
- Cannon, J.G.; Kraj, B.; Sloan, G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine 2011, 53, 141–144.
- Stilley, J.A.; Guan, R.; Duffy, D.M.; Segaloff, D.L. Signaling through FSH receptors on human umbilical vein endothelial cells promotes angiogenesis. J. Clin. Endocrinol. Metab. 2014, 99, E813–E820.
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of Follicle-Stimulating Hormone in Spermatogenesis. Front. Endocrinol. 2018, 9, 763.
- Santi, D.; Crepieux, P.; Reiter, E.; Spaggiari, G.; Brigante, G.; Casarini, L.; Rochira, V.; Simoni, M. Follicle-stimulating Hormone (FSH) Action on Spermatogenesis: A Focus on Physiological and Therapeutic Roles. J. Clin. Med. 2020, 9, 1014.
- Abel, M.H.; Baker, P.J.; Charlton, H.M.; Monteiro, A.; Verhoeven, G.; De Gendt, K.; Guillou, F.; O’Shaughnessy, P.J. Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology 2008, 149, 3279–3285.
- Tenuta, M.; Carlomagno, F.; Cangiano, B.; Kanakis, G.; Pozza, C.; Sbardella, E.; Isidori, A.M.; Krausz, C.; Gianfrilli, D. Somatotropic-Testicular Axis: A crosstalk between GH/IGF-I and gonadal hormones during development, transition, and adult age. Andrology 2021, 9, 168–184.
- Welsh, M.; Saunders, P.T.; Atanassova, N.; Sharpe, R.M.; Smith, L.B. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 2009, 23, 4218–4230.
- Huhtaniemi, I. A hormonal contraceptive for men: How close are we? Prog. Brain Res. 2010, 181, 273–288.
- Davies, A.G. Role of FSH in the control of testicular function. Arch. Androl. 1981, 7, 97–108.
- Hasenmajer, V.; Bonaventura, I.; Minnetti, M.; Sada, V.; Sbardella, E.; Isidori, A.M. Non-Canonical Effects of ACTH: Insights Into Adrenal Insufficiency. Front. Endocrinol. 2021, 12, 701263.
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581.
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211.
- Sowers, M.R.; Zheng, H.; McConnell, D.; Nan, B.; Harlow, S.; Randolph, J.F., Jr. Follicle stimulating hormone and its rate of change in defining menopause transition stages. J. Clin. Endocrinol. Metab. 2008, 93, 3958–3964.
- Robinson, L.J.; Tourkova, I.; Wang, Y.; Sharrow, A.C.; Landau, M.S.; Yaroslavskiy, B.B.; Sun, L.; Zaidi, M.; Blair, H.C. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem. Biophys. Res. Commun. 2010, 394, 12–17.
- El Khoudary, S.R.; Wildman, R.P.; Matthews, K.; Thurston, R.C.; Bromberger, J.T.; Sutton-Tyrrell, K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis 2012, 225, 180–186.
- El Khoudary, S.R.; Santoro, N.; Chen, H.Y.; Tepper, P.G.; Brooks, M.M.; Thurston, R.C.; Janssen, I.; Harlow, S.D.; Barinas-Mitchell, E.; Selzer, F.; et al. Trajectories of estradiol and follicle-stimulating hormone over the menopause transition and early markers of atherosclerosis after menopause. Eur. J. Prev. Cardiol. 2016, 23, 694–703.
- Munir, J.A.; Wu, H.; Bauer, K.; Bindeman, J.; Byrd, C.; Feuerstein, I.M.; Villines, T.C.; Taylor, A.J. The perimenopausal atherosclerosis transition: Relationships between calcified and noncalcified coronary, aortic, and carotid atherosclerosis and risk factors and hormone levels. Menopause 2012, 19, 10–15.
- Hopmans, S.N.; Duivenvoorden, W.C.; Werstuck, G.H.; Klotz, L.; Pinthus, J.H. GnRH antagonist associates with less adiposity and reduced characteristics of metabolic syndrome and atherosclerosis compared with orchiectomy and GnRH agonist in a preclinical mouse model. Urol. Oncol. 2014, 32, 1126–1134.
- Stefanska, A.; Sypniewska, G.; Ponikowska, I.; Cwiklinska-Jurkowska, M. Association of follicle-stimulating hormone and sex hormone binding globulin with the metabolic syndrome in postmenopausal women. Clin. Biochem. 2012, 45, 703–706.
- Song, Y.; Wang, E.S.; Xing, L.L.; Shi, S.; Qu, F.; Zhang, D.; Li, J.Y.; Shu, J.; Meng, Y.; Sheng, J.Z.; et al. Follicle-Stimulating Hormone Induces Postmenopausal Dyslipidemia Through Inhibiting Hepatic Cholesterol Metabolism. J. Clin. Endocrinol. Metab. 2016, 101, 254–263.
- Cui, H.; Zhao, G.; Liu, R.; Zheng, M.; Chen, J.; Wen, J. FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR. J. Lipid Res. 2012, 53, 909–917.
- Liu, X.M.; Chan, H.C.; Ding, G.L.; Cai, J.; Song, Y.; Wang, T.T.; Zhang, D.; Chen, H.; Yu, M.K.; Wu, Y.T.; et al. FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca(2+)/CREB pathway. Aging Cell. 2015, 14, 409–420.
- Liu, P.; Ji, Y.; Yuen, T.; Rendina-Ruedy, E.; DeMambro, V.E.; Dhawan, S.; Abu-Amer, W.; Izadmehr, S.; Zhou, B.; Shin, A.C.; et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 2017, 546, 107–112.
- Guo, Y.; Zhao, M.; Bo, T.; Ma, S.; Yuan, Z.; Chen, W.; He, Z.; Hou, X.; Liu, J.; Zhang, Z.; et al. Blocking FSH inhibits hepatic cholesterol biosynthesis and reduces serum cholesterol. Cell Res. 2019, 29, 151–166.
- Cannon, J.G.; Cortez-Cooper, M.; Meaders, E.; Stallings, J.; Haddow, S.; Kraj, B.; Sloan, G.; Mulloy, A. Follicle-stimulating hormone, interleukin-1, and bone density in adult women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R790–R798.
- Musabak, U.; Bolu, E.; Ozata, M.; Oktenli, C.; Sengul, A.; Inal, A.; Yesilova, Z.; Kilciler, G.; Ozdemir, I.C.; Kocar, I.H. Gonadotropin treatment restores in vitro interleukin-1beta and tumour necrosis factor-alpha production by stimulated peripheral blood mononuclear cells from patients with idiopathic hypogonadotropic hypogonadism. Clin. Exp. Immunol. 2003, 132, 265–270.
- Yousefi, S.; Karamlou, K.; Vaziri, N.; Carandang, G.; Ocariz, J.; Cesario, T. The effect of gonadotropins on the production of human interferon-gamma by mononuclear cells. J. Interferon Res. 1993, 13, 213–220.
- Komorowski, J.; Stepien, H. FSH and LH induce interleukin-6 (IL-6) release from human peripheral blood monocytes cultures in vitro. A dose-response study. Horm. Metab. Res. 1994, 26, 438–439.
- Syed, V.; Gerard, N.; Kaipia, A.; Bardin, C.W.; Parvinen, M.; Jegou, B. Identification, ontogeny, and regulation of an interleukin-6-like factor in the rat seminiferous tubule. Endocrinology 1993, 132, 293–299.
- Carbone, F.; Procaccini, C.; De Rosa, V.; Alviggi, C.; De Placido, G.; Kramer, D.; Longobardi, S.; Matarese, G. Divergent immunomodulatory effects of recombinant and urinary-derived FSH, LH, and hCG on human CD4+ T cells. J. Reprod. Immunol. 2010, 85, 172–179.
- Biffoni, M.; Marcucci, I.; Ythier, A.; Eshkol, A. Effects of urinary gonadotrophin preparations on human in-vitro immune function. Hum. Reprod. 1998, 13, 2430–2434.
- Deiktakis, E.E.; Ieronymaki, E.; Zaren, P.; Hagsund, A.; Wirestrand, E.; Malm, J.; Tsatsanis, C.; Huhtaniemi, I.T.; Giwercman, A.; Giwercman, Y.L. Impact of add-back FSH on human and mouse prostate following gonadotropin ablation by GnRH antagonist treatment. Endocr. Connect. 2022, 11, e210639.
- Dirnhofer, S.; Berger, C.; Hermann, M.; Steiner, G.; Madersbacher, S.; Berger, P. Coexpression of gonadotropic hormones and their corresponding FSH- and LH/CG-receptors in the human prostate. Prostate 1998, 35, 212–220.
- Ben-Josef, E.; Yang, S.Y.; Ji, T.H.; Bidart, J.M.; Garde, S.V.; Chopra, D.P.; Porter, A.T.; Tang, D.G. Hormone-refractory prostate cancer cells express functional follicle-stimulating hormone receptor (FSHR). J. Urol. 1999, 161, 970–976.
- Mariani, S.; Salvatori, L.; Basciani, S.; Arizzi, M.; Franco, G.; Petrangeli, E.; Spera, G.; Gnessi, L. Expression and cellular localization of follicle-stimulating hormone receptor in normal human prostate, benign prostatic hyperplasia and prostate cancer. J. Urol. 2006, 175, 2072–2077; discussion 2077.
- Gartrell, B.A.; Tsao, C.K.; Galsky, M.D. The follicle-stimulating hormone receptor: A novel target in genitourinary malignancies. Urol. Oncol. 2013, 31, 1403–1407.
- Beer, T.M.; Garzotto, M.; Eilers, K.M.; Lemmon, D.; Wersinger, E.M. Targeting FSH in androgen-independent prostate cancer: Abarelix for prostate cancer progressing after orchiectomy. Urology 2004, 63, 342–347.
More
