Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Danladi Chiroma Husaini and Version 2 by Jessie Wu.
Cereals are staple foods in Africa. The most commonly used cereals include maize (Zea mays), sorghum (Sorghum bicolor), millet (Peninsetum americanum), and acha or fonio (Digitaria exilis). These cereals are characterized by a high content of soluble non-starch polysaccharides (e.g., arabinoxylan and β–glucan), which have a health-promoting role and rich nondigestible carbohydrates (e.g., galacto- and fructooligosaccharides), excellent substrates for fermenting micro-organisms.
- cereals
- foods
- polysaccharides
1. Cereal-Based Fermented Foods
In sub-Saharan Africa, cassava (Manihot esculenta), also known by several regional names, is mainly a smallholder root crop, crucial for the food security of smallholder farmers, and primarily used to produce traditional fermented foods [1][9].
Fermentation of cereals is a common food processing practice performed at the household level for cereal preservation. It plays a crucial role in attaining food and nutrition security in sub-Saharan Africa [2][10]. Generally, fermentation positively affects the antioxidant activity of fermented grain-based foods through increased phenolic compounds, gamma-aminobutyric acid (GABA), and bioactive peptides. Changes in the vitamin contents of cereals due to fermentation vary according to the process conditions and the raw material used [3][11]. An increase in folate content is debated, and there is a current lack of information and research about the effect of lactic acid bacteria (LAB)-mediated fermentation on the vitamin content in fermented cereal foods [4][12]. Folate deficiency can cause severe deficiency during pregnancy, and there is evidence that insufficient intake of folic acid and cobalamin (vitamin B12) can drastically alter the immune system’s balance [5][13]. Saubade et al. (2018) [6][14] observed a relatively low folate content in ben-saalga, a pearl-millet-based fermented porridge from Burkina Faso, suggesting that folate is lost during the different processing steps. The step of spontaneous fermentation had no significant impact on folate content. Processing methods from different raw materials using corn, sorghum, and pearl millet have been shown to affect folate content and bioaccessibility in ben-saalga and six other African cereal-based fermented foods (akassa, doncounou, kaffafura, massa, and ben-kida). The main factors involved are the starting raw materials and traditional processing steps. Folate bioaccessibility is very variable and strongly influenced by the food matrix structure. Although the fermentation process positively affects the folate content in kaffa and akassa, the folate content is relatively low [7][15]. The optimum conditions for folate biosynthesis by LAB are still unclear, and optimizations are required to increase the LAB-mediated folate production in fermented food products [8][16]. Bacteria belonging to the genera Lactobacillus, Lactococcus, Leuconostoc, and Pedicoccus have been associated with cereal-based fermented foods. Other micro-organisms may be involved in fermentation processes, such as yeast species of Saccharomyces, Rhodotorula, Candida, Kluyveromyces, and Geotrichum genera [9][17], as well as filamentous molds belonging to the genera Aspergillus, Rhizopus, Fusarium, and Penicillium [10][11][8,18].
Pedersen et al. (2012) have identified C. krusei and K. marxianus as the dominant yeast species involved in the fermentation of fura, a spontaneously fermented pearl millet product consumed in West Africa. Both yeast species were capable of survival and growth under simulated gastrointestinal conditions and the transepithelial electrical resistance (TEER) of the human Caco-2 cell line, suggesting a potential probiotic property of these yeasts [12][19]. Owusu-Kwarteng et al. (2015) [13][20] obtained similar results, reporting the in vitro probiotic properties of sixteen Lactobacillus fermentum strains isolated from West African fermented millet dough. Interestingly, four L. fermentum strains showed antibacterial activity against Listeria monocytogenes NCTC 10527 and Staphylococcus aureus ATCC 1448. The yeast Pichia kudriavzevii strain isolated from ogi (a traditional, spontaneously maize-based fermented food from Benin) increased the production of folate (vitamin B9) when inoculated in pearl millet (Pennisetum glaucum)-based gruel with L. fermentum [14][21]. Yeast strains of K. marxianus and S. cerevisiae isolated from West Africa fermented dairy foods lait caillé and nunu and a cereal-based food mawè exhibited the highest probiotic potential [15][22]. Imade et al. (2021) [16][23] isolated four LAB strains identified as Limosilactobacillus fermentum NBRC15885, Limosilactobacillus fermentum CIP102980, Companilactobacillus nantensis LP33, and Lactiplantibacillus garii JCM1149 and isolated from fufu, nono, ogi, and kunu showed the ability to synthesize bacteriocin actives against pathogenic strains of B. cereus, Klebsiella pneumonia, and S. typhimurium. Bacteriocin is a ribosomally synthesized antimicrobial peptide that can exert a bioprotective effect against many food-spoilage and food-borne pathogenic bacteria, such as Staphylococcus aureus, Listeria monocytogenes, Bacillus cereus, and Clostridium botulinum [17][24].
Cereal-based fermented beverages are prevalent in Africa, and fermented food beverages are a rich source of bioactive compounds [18][25]. In Ethiopia, there is a long oral tradition about indigenous fermented beverages produced from different cereal raw materials, such as barley, maize, wheat, and honey. These beverages are mainly produced by acid and alcoholic fermentation (i.e., mediated by mixed cultures of micro-organisms, such as LAB and yeasts) [19][20][26,27]. Togwa (from maize flour, finger millet malt, Tanzania), a sweet and sour, nonalcoholic beverage, is one of the better-studied African cereal beverages. A different maize-based laboratory model of togwa showed how yeasts isolated from Tanzanian fermented food togwa can significantly increase folate content in the fermented product [21][28]. The yeast Pichia kudriavzevii isolates also showed potential probiotic abilities [14][21].
Several authors have described the potential role of food fermentation processes in reducing toxic compounds in raw food materials by harnessing autochthonous microflora involved in traditional fermentation processes or by adding selected starter cultures and adopting controlled fermentation. While few studies have documented an increase in mycotoxin levels after food fermentation processes, a decrease in mycotoxin levels has been generally reported [22][29]. In particular, the role of probiotics in mycotoxin biodetoxification has been described. Several micro-organisms have been reported to reduce mycotoxin accessibility, adsorption, and biotransformation in the gut through different mechanisms, including Lactobacillus, Bifidobacterium, and some Bacillus species of yeast Saccharomyces cerevisiae [23][30].
If fermentation processes seem to reduce mycotoxins levels in the final product (compared to the raw materials), traditionally processed beverages should benefit from adopting a value chain approach. Such an approach should include practical solutions to reduce mycotoxin exposure, such as educational interventions and grain-cleaning methods to optimize processing conditions/steps [24][31]. Recently, Nafuka et al. (2019) [25][32] highlighted the importance of monitoring emerging mycotoxins, aflatoxin precursors, and ergot alkaloids in sorghum malts used to produce Namibian traditional fermented sorghum-based beverages, omalodu and otombo. Indeed, the growth of mycotoxigenic fungi can be stimulated under warm, moist, and likely unhygienic conditions that may occur during the malting and milling processes.
The LAB and yeasts can also reduce exposure to various chemical food contaminants, including metals, metalloids, and cyanotoxins [26][33]. The content of cyanogenic glycosides lotaustralin and linamarin is reduced in African-fermented cassava products, such as gari and fufu, during cassava fermentation by Lactobacillus, Streptococcus, and Leuconostoc [27][34]. The LAB potentially detoxify heavy metals in foods through biosorption, bioaccumulation, and transformation [28][35]. The detoxification of heavy metals further enhances the potential probiotic relevance of LAB, many of which are found in several Nigerian fermented foods [29][36].
2. Meat- and Fish-Based Fermented Foods
Numerous meat-based fermented foods are also present in many traditional African diets, especially in the Mediterranean [30][37]. Together with dairy products, fermented meat products are also sources of peptides with antioxidant activity [3][11]. Recent studies have focused on the identification and quantification of bioactive peptides derived from fermented meat products and their possible roles in disease prevention [31][38].
Several studies have reported microbiological characterizations of fermented meat products from North Africa. For instance, Belgacem et al. (2010) [32][39] isolated 24 strains of Enterococcus faecium from gueddid, a traditionally Tunisian fermented meat, producing bacteriocin production with inhibitory activity against Listeria spp., Enterococcus spp., and Staphylococcus aureus. One isolate was active against Escherichia coli CECT 877. Nine of the antagonistic enterococci tested did not show any virulence traits or produce biogenic amines. Despite the probiotic potential of the genus Enterococcus, as well as their contribution to the ripening and aroma development of fermented meat products, the prevalence of virulence factors and antibiotic-resistance genes and their ability to cause disease can pose risks for food safety issues [33][40]. Thus, to properly evaluate food safety in traditional fermented meat productions, the monitoring of Enterococcus strains should be encouraged.
Boudechicha et al. (2017) [34][41] provided a preliminary microbiological characterization of khliaa ezir, Algeria’s traditional cured meat product. The LAB are the most bountiful in the product during the ripening and storage. A low level of enterobacterial population and a high general hygiene quality have been attributed to the spicing and salting thermal treatment steps. Similar results have been obtained by Benlacheheb et al. (2019) from a microbiological study on el-guedid, an Algerian traditional fermented red meat-based product [35][42]. Aerococcus and Enterococcus species isolated from el-guedid have exhibited a probiotic potential [36][43]. Bader et al. (2021) reported the results of a more comprehensive study of el-guided physicochemical and microbiological properties, considering the type of raw red meat and the conservation time [37][44]. Lactic acid bacteria and coagulase-negative staphylococci were the dominant populations in el-guedid, including Leuconostoc mesenteroides, Lactobacillus sakei, and Staphylococcus saprophyticus. In particular, L. sakei and L. mesenteroides can produce bacteriocins that could contribute to the microbiological safety of el-guedid. Bacteria, including LAB and coagulase-negative staphylococci, can increase the safety of fermented meat products by controlling or reducing the microbiological hazards of bacteriocin production [38][45]. Generally, bacteriocins have shown a tremendous inhibitory effect on Listeria monocytogenes in meat products [39][46]. Bacteriocin production could have a protective culture in fermented meats for the control or reduction in microbiological hazards; however, bacteriocins may inhibit desired starter cultures and may not be active against food spoilage bacteria [40][47].
Fermentation is also a widespread practice for fish preservation in Africa. Prominent examples are lanhouin (Benin and Togo), momone, koobi, kako, and ewule (Ghana), guedj (Gambia), tambadiang, and guedj (Senegal), djege and jalan (Mali), fessiekh, kejeick, terkeen, and mindeshi (Sudan), dagaa (Uganda), gyagawere, adjonfa, and adjuevan (Côte d’Ivoire), and salanga (Chad) [41][48]. In local cereal-based diets, fermented fish products are generally used as taste- and flavor-enhancing condiments or as a source of animal proteins. The production of traditional fish-based fermented foods is based chiefly on spontaneous fermentation processes. As for many other traditional food fermentations, such food processing techniques would require more attention both in terms of standardization of the operations and improvement in hygienic aspects [42][43][44][49,50,51]. The LAB and yeasts are the dominant micro-organisms in many fermented fish products [45][52]. Many yeast and bacterial strains have been isolated from momoni, a Ghanaian fermented fish condiment, with Bacillus species predominant [46][53]. However, authors have suggested that the fermentation process is mediated by the endogenous fish enzymes rather than the associated microflora due to the high pH and high salt concentrations. Farag et al. (2022) have suggested that future studies will be required to understand the better microbial impact on the quality of fermented and salted fish such as feseekh, moloha, and renga from Egypt [47][54].
Lanhouin is a fermented fish-based product widely used as a condiment in Benin, Togo, and Ghana. Lanhouin is processed by spontaneous fermentation from different fishes, such as cassava croaker/cassava fish (Speudotolithus sp.) or Spanish mackerel/kingfish (Scomberomorus tritor), and different processes as well. Anihouvi at al. (2007) [48][55] have reported changes in microbial communities during spontaneous fermentation of lanhouin from cassava fish (Pseudotolithus sp.). Bacillus subtilis, Bacillus licheniformis, Staphylococcus lentus, and Staphylococcus xylosus persisted up to the end of fermentation.
Koffi-Nevry et al. (2011) [49][56] have studied the LAB communities in adjuevan, a traditional salted fermented fish (the Atlantic bumper, Chloroscombrus chrysurus) from Côte d’Ivoire. Lactobacillus fermentum, Leuconostoc lactis, Pediococcus sp., and Streptococcus sp. have been isolated both from the fresh fish Chloroscombrus chrysurus and the adjuvant samples. Similarly, adjuvant microbial community dynamics were produced using the whole fish and fish fillets [50][57]. Although the composition varied according to the preparation method, yeast, and several LAB communities were found for both. LAB included Lactobacillus, Pedicoccus, Lactococcus, Streptococcus, and Leuconostoc species, but no LAB species were dominant. Clémentine et al. (2020) [51][58] have instead studied yeast diversity in adjuevan fermentation. Seven species of yeast have been identified, and varied fermentation methods and salt concentrations used. These include Pichia fermentans, Candida zeylanoides, Candida sp., Hanseniaspora osmophila, Kluyveromyces sp., Torulaspora delbrueckii, and Kluyveromyces marxianus. All these yeast species have probiotic potential [52][59].
3. Dairy Fermented Products
With its substantial social and cultural value, milk has always been a critical dietary component in sub-Saharan pastoral communities [53][54][60,61].
As for other fermented foods, fermentation was primarily used as a traditional food preservation practice for surplus milk produced during the wet season. Regarding their health-promoting properties, yogurt and fermented milk have higher antioxidant activity than milk because of the release of bioactive peptides by microbial-mediated proteolysis. Dairy products are rich sources of bioactive peptides with several activities, including immunomodulatory and antioxidant properties [31][38]. Several factors can influence the antioxidant power of fermented milk, such as milk origin, milk fat content, and the presence and position in milk peptides of the amino acids tryptophan, tyrosine, methionine, and fermenting micro-organism strains [55][62]. Increased formation of conjugated linoleic acid (CLA) and folates in fermented milk can also increase antioxidant power [3][11]. Dairy products are excellent sources of vitamin B12, mainly synthesized by anaerobic micro-organisms [56][63]. Another vitamin essential for human health is the lipid-soluble vitamin K. In particular, one of the two biologically active forms of vitamin K, vitamin K2, is predominantly of microbial origin and refers to a group of menaquinones (MKs) with different side chain lengths. Long-chain vitamin K2 forms are produced by food-grade bacteria involved in food fermentation processes, such as Bacillus subtilis and, interestingly, by some species and strains of LAB, such as Lactococcus lactis ssp. Cremoris, L. lactis ssp. Lactis, Leuconostoc lactis, and Leuconostoc mesenteroides [57][64]. Vitamin K2 can also be synthesized by bacteria belonging to the Bacteroides genus, one of the two most important gut microbiota genera. However, due to its poor bioavailability, the major source of functionally available vitamin K2 is the diet. Therefore, dairy products may be a predominant source of dietary MK in many regions of the world. Recently, there has been considerable interest in enhancing the MK content of dairy products through the identification and selection of MK-producing bacteria in dairy fermentations [58][65].
Fermented milk is the most widely fermented dairy product in traditional African diets. Some examples include spontaneously fermented milk, such as iben (Algeria, North Africa), sussa (Ethiopia, Somalia, Kenya, and Sudan), ergo (Ethiopia), kule and amabere amaruranu (Kenya), kivuguto (Rwanda), amasi (Zimbabwe), and nunu (Ghana). Inoculated fermentation methods, in some cases performed as semicontinuous or fed-batch fermentation processes, are also practiced. Even if not properly standardized, such inoculated fermentation methods can contribute to the stabilization of production processes. Some examples include zabady (Egypt), rob, biruni, garris (Sudan), masse (Mozambique), madila (Botswana), and omashikwa (Namibia). The preparation of fresh and ripened cheeses is also part of traditional diets [59][66].
The LAB, such as Lactococcus lactis, Streptococcus infantarius subsp. Infantarius and Lactobacillus spp. dominate African fermented dairy productions mainly as autochthonous milk microflora and indigenous microbial flora, brought by utensils and containers used for milk preservation [60][67]. Mesophilic bacteria (Lactococcus and Leuconostoc spp.) in fermented dairy foods are found mainly in cold climatic regions, while thermophilic bacteria (Lactobacillus and Streptococcus spp.) are found in hot climatic regions. Yeasts are often associated with fermented dairy products, e.g., amabere amaruranu, gariss, nunu, and rob [61][68]. Lack of standardization procedures and scarce hygiene conditions that often occur in milk production and processing can affect fermented dairy products’ shelf stability and pose risks to consumers [62][63][64][65][69,70,71,72]. A recent review about pathogenic and chemical contaminants in dairy products across sub-Saharan Africa highlighted current gaps as well as the need for robust investigations into these food safety risks [66][73].
The LAB present in dairy fermented products can exert bioprotection against food spoilage and food-borne pathogenic bacteria (e.g., Staphylococcus aureus, Escherichia coli, Campylobacter jejuni, and Vibrio cholera), mainly through a pH reduction that occurs during fermentation. Todorov (2008) [67][74] reported the production of bacteriocins active against Listeria, produced by Lactobacillus Plantarum isolated from amasi (a naturally fermented milk product from Zimbabwe). Moshba et al. (2018) have reported the production of bioactive peptides from the proteolysis of whey proteins in camel milk with antimicrobial activity against S. aureus, P. aeruginosa, K. pneumoniae, and E. coli [68][75]. The inhibitory effect of camel milk against pathogenic micro-organisms is well known [69][76]. In addition, several bioactive compounds (lactoferrin, α-lactalbumin, β-caseins and vitamin C, lysozyme, IgG and secretory IgA, and insulin-like protein) present in camel milk exhibited antioxidant, anti-inflammatory and immunomodulatory properties [70][77].
Rural dairy processing can also contribute to the protection of the final product against food-borne pathogenic bacteria. Two examples are the production of smoked vessels for the Ethiopian fermented milk ergo that can slow the growth of coliforms or the use of flavors, such as black cumin in mish, a ripened soft cheese from Egypt and Sudan [59][66]. Most artisanal cheeses in East Africa and Northern Africa are soft cheeses [71][78]. Some examples of fresh cheeses include klila, warankasi, kariesh (or karish), ayib, and gibna. In North Africa, hard-ripened cheeses (such as domiati, mish, and bouhezza) are more common than in other parts of Africa [59][66]. Some exceptions include touaregh (Mali) and tchoukou (Niger) [71][78]. Traditional cheese production at the rural level is generally characterized by scarce hygiene standards favored by a lack of cheese-making standardization in safety aspects managed empirically. Abdelfatah and Tahoun (2015) studied LAB in Kariesh cheese, and a variety of LAB have been isolated. In particular, Lactobacillus rhamnosus was the most prominent strain in kariesh cheese, and the high antibiotic-resistant Lactococcus garvieae pathogenic strain was isolated [72][79]. The probiotic potential of Pediococcus acidilactici isolated from wara has been recently investigated and reported [73][80]. Tchoukou cheese (Niger) is a rich source of calcium and zinc with high nutritional value [74][81]. A recent evaluation reported the probiotic potential of lactic acid bacteria strains isolated from tchoukou cheese [75][82]. The LAB Lactobacillus fermentum, Lactobacillus intestinalis, and Lactobacillus acidophilus isolated from klila cheese exhibited strong bactericidal activity against S. aureus [76][83].
Marino et al. 2012 [77][84] conducted a study to characterize healthy beneficial compounds in bouhezza cheese. Results showed how raw milk quality and cheese-making technology could strongly affect fat-soluble antioxidants, linolenic acid, and conjugated linoleic acid contents.
Results about traditional fermented foods with potential health benefits are presented in Table 1 and Figure 1.
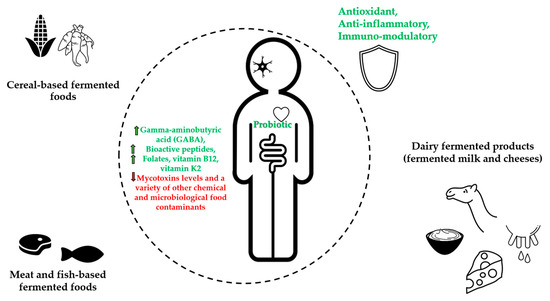
Figure 1.
Graphical illustration of the main health effects from African traditional fermented foods.
Table 1.
Traditional African fermented foods with potential health benefits, including the strength of the immune system.
| Fermented Foods | Raw Food Materials | Micro-Organisms | Bioactive Compounds |
Potential Health Benefits |
References |
|---|---|---|---|---|---|
| Cereal-based fermented foods |
Maize (Zea mays), Sorghum (Sorghum bicolor), Millet (Peninsetum americanum), Acha or Fonio (Digitaria exilis), Cassava (Manihot esculenta) |
Bacteria (Lactobacillus, Lactococcus, Leuconostoc Pedicoccus genera) Yeasts (Saccharomyces, Rhodotorula, Candida, Kluyveromyces, and Geotrichum genera) Filamentous molds (Aspergillus, Rhizopus, Fusarium, and Penicillium, genera) |
Soluble non-starch polysaccharides (e.g., arabinoxylan and β–glucan) Nondigestible carbohydrates (e.g., galacto- and fructo–oligosaccharides) Folates |
Promote rich nondigestible carbohydrates (prebiotics), increase in phenolic compounds, gamma-aminobutyric acid (GABA), and bioactive peptides contents. Increase folates, decrease mycotoxins levels, increase health benefits of probiotic consumption, reduce exposure to a variety of other chemical food contaminants and detoxification |
[9][10][11][22] [26][28][29] |
| Meat- and fish-based fermented foods | Meat, Fish |
Bacteria (Leuconostoc, Lactobacillus, Enterococcus Aerococcus, Bacillus genera) Yeasts (Pichia, Candida, Hanseniaspora, Kluyveromyces Torulaspora, and Kluyveromyces genera) |
Bioactive peptides, Bacteriocins |
Antioxidant activity, increase health benefits of probiotic consumption, reduction of microbiological hazards | [3][31][38] [45][52] |
| Dairy fermented products (fermented milk and cheeses) | Milk | Bacteria (Lactococcus Leuconostoc Streptococcus, Lactobacillus, Pediococcus genera) Yeasts (Saccharomyces, Candida, Kluyveromyces genera) |
Bioactive peptides, Conjugated Linoleic Acid, Vitamin B12, Vitamin K2 Bacteriocins |
Antioxidant, immunomodulatory, source of vitamin B12 and vitamin K2, increase health benefits of probiotic consumption, protection against food-spoilage |
[3][31][54][58] [61] |
| Fermented Foods | Raw Food Materials | Micro-Organisms | Bioactive Compounds |
Potential Health Benefits |
References |
|---|---|---|---|---|---|
| Cereal-based fermented foods |
Maize (Zea mays), Sorghum (Sorghum bicolor), Millet (Peninsetum americanum), Acha or Fonio (Digitaria exilis), Cassava (Manihot esculenta) |
Bacteria (Lactobacillus, Lactococcus, Leuconostoc Pedicoccus genera) Yeasts (Saccharomyces, Rhodotorula, Candida, Kluyveromyces, and Geotrichum genera) Filamentous molds (Aspergillus, Rhizopus, Fusarium, and Penicillium, genera) |
Soluble non-starch polysaccharides (e.g., arabinoxylan and β–glucan) Nondigestible carbohydrates (e.g., galacto- and fructo–oligosaccharides) Folates |
Promote rich nondigestible carbohydrates (prebiotics), increase in phenolic compounds, gamma-aminobutyric acid (GABA), and bioactive peptides contents. Increase folates, decrease mycotoxins levels, increase health benefits of probiotic consumption, reduce exposure to a variety of other chemical food contaminants and detoxification |
[8,17,18,29, 33,35,36] |
| Meat- and fish-based fermented foods | Meat, Fish |
Bacteria (Leuconostoc, Lactobacillus, Enterococcus Aerococcus, Bacillus genera) Yeasts (Pichia, Candida, Hanseniaspora, Kluyveromyces Torulaspora, and Kluyveromyces genera) |
Bioactive peptides, Bacteriocins |
Antioxidant activity, increase health benefits of probiotic consumption, reduction of microbiological hazards | [11,38,45, 52,59] |
| Dairy fermented products (fermented milk and cheeses) | Milk | Bacteria (Lactococcus Leuconostoc Streptococcus, Lactobacillus, Pediococcus genera) Yeasts (Saccharomyces, Candida, Kluyveromyces genera) |
Bioactive peptides, Conjugated Linoleic Acid, Vitamin B12, Vitamin K2 Bacteriocins |
Antioxidant, immunomodulatory, source of vitamin B12 and vitamin K2, increase health benefits of probiotic consumption, protection against food-spoilage |
[11,38,61,65 68] |
