Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Hristo Petkov and Version 2 by Catherine Yang.
1-amidoalkyl-2-naphthol derivatives are of increasing interest due to their biological activities and further use in the preparation of other important bioactive molecules, such as aminoalkyl naphthols and oxazines.
- 1-amidoalkyl-2-naphthol
- bioactivity
- aminoalkyl naphthol
- oxazine
1. Introduction
The 1,3-aminooxygenated functional motif exists in a variety of natural products [1][2][3][4][1,2,3,4] as well as in a number of marketed drugs [5][6][7][8][9][10][5,6,7,8,9,10]. Sedridine, allosedridine, febrifugine, nikkomycin Z, and negamycin, bearing 1,3-aminooxygenated moiety, are naturally occurring compounds some of which possess antifungal and antibacterial properties [1][2][3][4][1,2,3,4]. Ritonavir and lopinavir, approved antiretroviral drugs against HIV/AIDS [5][6][5,6]; haloperidol, an antipsychotic drug [7]; venlafaxine and desvenlafaxine, antidepressant medications [8]; vildagliptin, an antidiabetic drug [9]; and tramadol, a synthetic analgesic [10], are marketed drugs which also feature the 1,3-aminooxygenated motif. As can be seen, the list of bioactive molecules containing the 1,3-aminooxygenation pattern is fairly long, which justifies and encourages the search for other valuable and pharmaceutically interesting compounds bearing this functional motif.
Naphthalene compounds are known to exhibit diverse biological activities. They possess anti-inflammatory [11], antibacterial [12][13][14][12,13,14], cardiovascular [15], antiproliferative [16], and antiviral [17] properties. A pharmaceutically interesting class of substances is the 1-amidoalkyl-2-naphthols, which possess the important 1,3-aminooxygenated moiety in their molecular structures, as well as an amide linkage, along with a naphthalene ring (Figure 1).
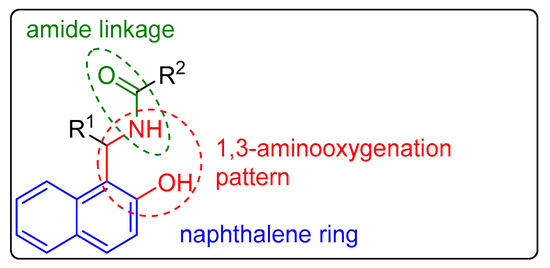
Figure 1.
General structure of 1-amidoalkyl-2-naphthols.
2. Amidoalkyl Naphthols as Bioactive Molecules
The 1-amidoalkyl-2-naphthols possess diverse biological properties, such as antiviral, antibacterial, antifungal, and antiparasitic qualities [18][19][20][21][22][18,19,20,21,22]. Furthermore, members of this family are promising antioxidants, which also show cholinesterase and α-glucosidase inhibitory activities [23][24][25][23,24,25].
Abou-Elmagd and colleagues evaluated the in vitro antibacterial and antiviral activities of a series of amidoalkyl naphthol derivatives featuring pyrazole or indole fragments [18]. The most potent compounds are illustrated in Figure 2. The results of the antibacterial test revealed that compounds 1 and 4 show high potency against Staphylococcus aureus, whereas compounds 2 and 3 have more pronounced inhibitory effects towards Klebsiella pneumoniae and Bacillus subtilis, respectively. All four derivatives demonstrated comparable antibacterial activities to the used reference, gentamicin. In terms of antiviral properties, the most active compounds against avian influenza virus (H5N1) were found to be 3 and 5, which showed similar results to the control, zanamivir.
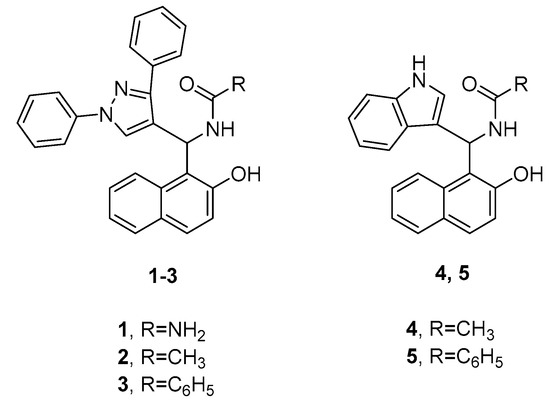
Figure 2.
Representative amidoalkyl naphthol derivatives with different antibacterial and antiviral properties.
Bananezhad and co-workers synthesized a series of 14 amidoalkyl naphthols bearing an azo-moiety and subjected them to in vitro antimicrobial assays [19]. It was observed that none of the derivatives showed better antibacterial activities than the reference drugs trimethoprim/sulfamethoxazole (SXT) and ciprofloxacin. However, the results from the antifungal test were very promising, since five substances (compounds 6–10, Figure 3) exhibited better potencies against Aspergillus niger than the standard drugs fluconazole and miconazole.
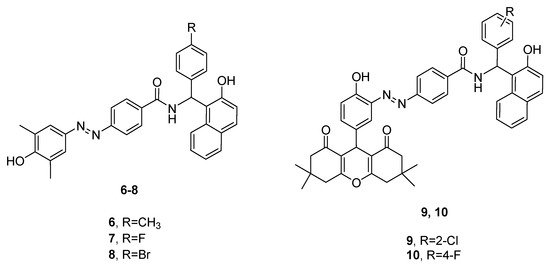
Figure 3.
Antifungal amidoalkyl naphthols.
Another study by Manavi et al., revealed the in vitro anti-Helicobacter pylori activities of various amidoalkyl naphthols [20]. The obtained results illustrated that many of them show promising potency with compound 11 (Figure 4) being the most active against this bacteria.
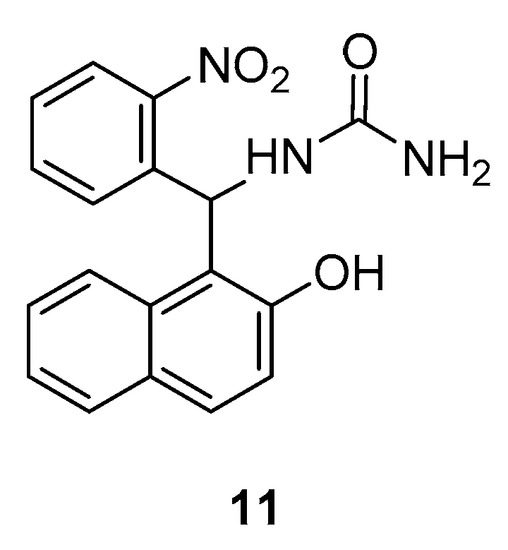
Figure 4.
Promising anti-
Helicobacter pylori
agent from the amidoalkyl naphthol class.
Rahimizadeh et al., used different benzaldehydes and long-chain amide derivatives to prepare a number of amidoalkyl naphthols, and examined their antibacterial properties in vitro [21]. The results indicated that compounds 12–18 (Figure 5) were the most potent against Staphylococcus aureus, exhibiting better activity than the reference, gentamicin.
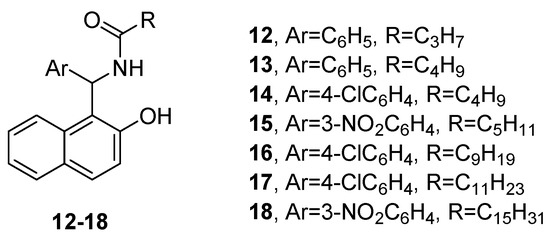
Figure 5.
Amidoalkyl naphthols potent against
Staphylococcus aureus.
Rode and co-authors reported the antiparasitic activity of a series of 23 amidoalkyl naphthol derivatives [22]. As a result of an in vitro screening against Leishmania donovani, followed by a molecular docking study and an in silico study of the ADME (absorption, distribution, metabolism, and excretion) properties of the molecules, four of them (compounds 19–22, Figure 6) were distinguished as the most promising anti-leishmanial agents possessing good drug-like characteristics.
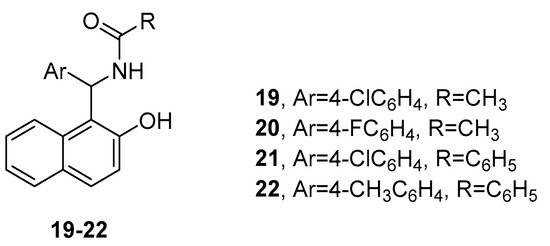
Figure 6.
Amidoalkyl naphthols with antiparasitic properties.
Boudebbous et al., synthesized 19 amidoalkyl naphthols and evaluated their cholinesterase and α-glucosidase inhibitory activities [23]. The results of the in vitro assays were backed by a docking study of selected derivatives. The properties of compounds 23 and 24 (Figure 7) were found to be very promising since both substances showed better inhibition on cholinesterase than the reference drug galantamine. Derivative 24 was also the most potent one in the α-glucosidase assay and exhibited a greater inhibitory effect than quercetin and acarbose, which were used as standards.
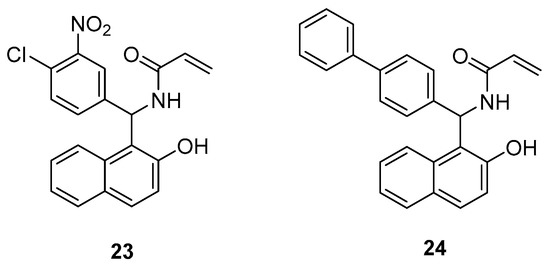
Figure 7.
Amidoalkyl naphthols proven as potent cholinesterase and α-glucosidase inhibitors.
In vitro and in silico studies by Boudebbous et al., aimed to estimate the biological potential of another two amidoalkyl naphthol derivatives—NDHA [24] and BHMA [25] (Figure 8). Both compounds exhibited promising antioxidant properties, and in addition, NDHA was proven to be a potent acetylcholinesterase and α-glucosidase inhibitor.
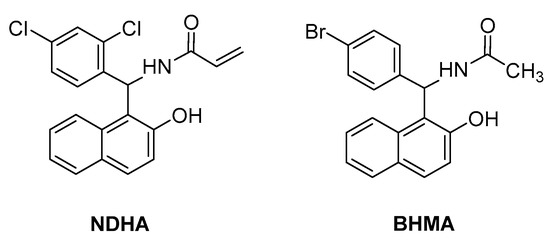
Figure 8.
Amidoalkyl naphthols with promising antioxidant properties.
Based on the pronounced biological activities that amidoalkyl naphthols exhibit, it can be concluded that this class of compounds has the potential to become an important starting point for the pharmaceutical industry. However, more in-depth studies are needed in order to evaluate the physiological effect of these substances.
3. Amidoalkyl Naphthols as Building Blocks
As well as possessing interesting properties, amidoalkyl naphthols are valuable building blocks for the preparation of other bioactive molecules. They can be easily converted to aminoalkyl naphthols, the so-called Betti bases, via hydrolysis or reduction [23][26][27][28][29][23,26,27,28,29] (Scheme 1). These are of great importance because of their biological activities, such as enhancing antitumor agents’ cytotoxicity [30], and hypotensive and bradycardiac effects [31]. In addition, these compounds can be used as ligands to chelate with organometallic reagents in asymmetric synthesis and catalysis [32][33][34][32,33,34].
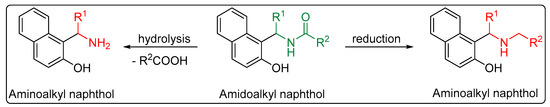
Scheme 1.
Hydrolysis and reduction of amidoalkyl to aminoalkyl naphthols.
Furthermore, the intramolecular cyclization of amidoalkyl naphthols produces 1,3-oxazines via the Vilsmeier–Haack reaction (Scheme 2). These compounds have also attracted interest because of their potential as antibiotics, antitumor agents, analgesics, and anticonvulsants [35].
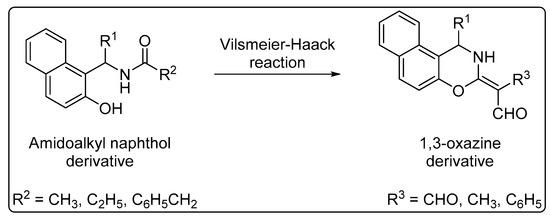
Scheme 2.
Intramolecular cyclization of amidoalkyl naphthols to 1,3-oxazines.
4. Synthesis of Amidoalkyl Naphthols via Multicomponent Mannich Condensation
Although many routes to obtain similar structures including phenols and naphthols, e.g., via transition-metal catalysis, have been reported [36][37][38][39][38,39,40,41], multicomponent Mannich condensation continues to be the primary way to achieve the synthesis of amidoalkyl naphthols.
Multicomponent reactions are of increasing importance in organic synthesis for the preparation of complex and diverse molecules through the formation of carbon–carbon and carbon–heteroatom bonds. These reactions involve three or more compounds mixed simultaneously in one vessel that react with each other and give the final target product without having to isolate the intermediates. Hence, one-pot multicomponent synthesis is in accordance with the principles of green chemistry, which require the development of new substances and reaction conditions that have the potential to provide benefits to chemical synthesis to meet certain requirements regarding resource and energy efficiency, operational simplicity, and product selectivity, as well as including environmental and health protections through reducing toxic solvent use and amount of waste produced as much as possible [40][41][42][43][44][42,43,44,45,46]. An example of such a reaction is the Mannich condensation of 2-naphthol with aldehydes and amide derivatives, in the presence of a catalyst, which is used as a practical synthetic route towards 1-amidoalkyl-2-naphthols [45][47] (Scheme 3). The same reaction is applicable to the synthesis of 1-carbamatoalkyl-2-naphthols when carbamates are used as reaction partners [46][47][48][49][50][51][48,49,50,51,52,53]. However, the reports on the synthesis of 1-carbamatoalkyl-2-naphthols over the last 3 years are limited [52][53][54,55]. The generally accepted reaction mechanism of the acid-promoted three-component Mannich condensation of 2-naphthol with aldehydes and amides proceeds with the initial formation of highly reactive ortho-quinone methides. These intermediates react with an amide via nucleophilic conjugate addition to form 1-amidoalkyl-2-naphthol (Scheme 3) [54][55][56][56,57,58].
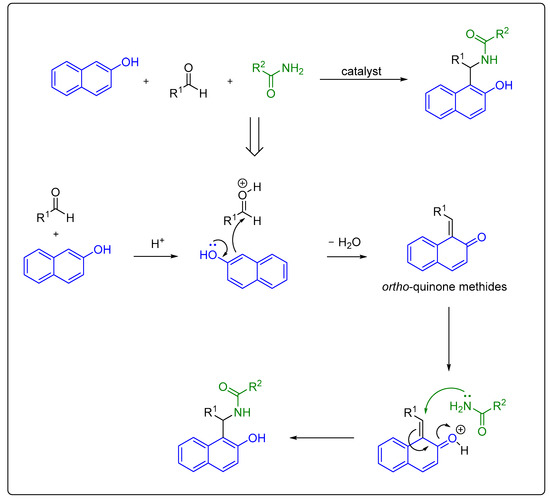
Scheme 3.
Synthesis of amidoalkyl naphthols via multicomponent Mannich condensation and plausible mechanism.
