Solid lipid nanoparticles are just a solid core of lipids formed by one of the following techniques at 37 °C: high-pressure homogenization, double emulsion, high-shear homogenization, or emulsifier evaporation. The lipid core can be then functionalized using a PEGylated layer or by alternate adsorption. Solid lipid nanoparticles either have one drug incorporated inside their core during fabrication, or they may contain more than one drug. In general, both hydrophobic and hydrophilic drugs can be inserted. For instance, solid lipid nanoparticles are characterized by biocompatibility, low toxicity, good stability, and enhancement of entrapped lipophilic drugs.
Various nanocarriers have obtained great advantages in diverting the drugs into aerosols and providing highly sustainable nebulization forces, and these have been used recently in the treatment of lung cancer [44]. For this reason, many nanoparticles have been designed and developed for the treatment of lung cancer, such as liposomes, micelles, solid lipid nanoparticles, and polymeric nanoparticles [45]. Indeed, lipid-based NPs and polymeric nanoparticles (PNPs) are considered to be successful delivery systems. These assemblies have been accepted by the United States Food and Drug Administration (FDA) [46]. Lipid-based nanocarriers can be further classified into liposomes, solid lipid nanoparticles, and hybrid polymeric lipid nanoparticles.
As a comparison between liposomes, solid lipid nanoparticles and hybrid polymeric lipid nanoparticles, it can be summarized that liposomes are mostly coformulated by the presence of a phospholipid and cholesterol core and shell in their structure [101]. Their diameters depend mainly on the method of fabrication, surfactants, concentrations of phospholipids and cholesterol, dehydration time, and temperature. The architecture of liposomes has obtained much interest in terms of encapsulating hydrophilic drugs inside the core and hydrophobic drugs inside the shell [102]. Hence, this assembly is characterized by the encapsulation of more than one type of drug with different physicochemical properties. Additionally, the spherical bilayer system causes the barrier to prevent the leakage of drugs out of liposome moieties [103].
In this way, liposomes have great advantages as biodegradable and biocompatible materials with the potential for large-scale production during fabrication, allowing them to be used widely in biomedical applications. Besides that, entrapped drugs can be modulated inside the core and shell.
The disadvantage of liposomes is mostly attributed to the possible oxidation of the liposomal phospholipid layer, leading to hydrolysis of the membrane and degradation of the liposomal structure. This drawback leads to the leakage of drugs out of the liposome. Furthermore, lipid peroxidation leads to damage to the properties of the lipid structure, particularly cellular permeability. Additionally, temperature, pH, and light may induce instability into the physical and chemical properties of liposomes, leading to a reduction in the shelf life of liposomes during long-term storage. The other disadvantages could be related to the use of the freeze-drying technique during liposomal fabrication [104], since this technique leads to the rupture of phospholipid membranes. To overcome these drawbacks, liposomes are mostly coated with polymeric materials, such as a PEGylated layer, during fabrication or incorporated inside CaCO3 using the layer by layer technique.
Solid lipid nanoparticles are just a solid core of lipids formed by one of the following techniques at 37 °C: high-pressure homogenization, double emulsion, high-shear homogenization, or emulsifier evaporation [105]. The lipid core can be then functionalized using a PEGylated layer or by alternate adsorption [106]. Solid lipid nanoparticles either have one drug incorporated inside their core during fabrication, or they may contain more than one drug [107]. In general, both hydrophobic and hydrophilic drugs can be inserted. For instance, solid lipid nanoparticles are characterized by biocompatibility, low toxicity, good stability, and enhancement of entrapped lipophilic drugs.
The disadvantage of this structure is mostly associated with the type of lipid used, as this can produce cytotoxicity related to free fatty acids and can cause a growth in the diameter during fabrication [108]. Additionally, the diameter grows during the fabrication of micro- and macroparticles [109].
Polymeric nanoparticles can be identified as an interaction of two opposite polymeric materials that form a cross-linked network and condensed core [110]. This core can be further functionalized by a PEGylated layer or coated by a lipid monolayer. Hanafy et al. optimized hybrid polymeric lipid nanoparticles by using chitosan-oleic acid after blocking its free fatty acid [111]. This strategy reduced the cytotoxicity that can be generated by oleic acid. Hybrid polymeric lipid nanoparticles can be co formulated in the shape of micelles by using polymer self-assembly, while drugs can either be attached during fabrication or inserted after fabrication. Recently, polymeric materials in the shape of hydrogel materials, mucoadhesive materials, and stimuli-responsive polymers have been developed. Polymeric nanoparticles are characterized by controlled drug release, stability inside cells, and easy and cost-effective formulation. However, the disadvantage of polymeric NPs is mostly associated with the type of organic solvent used during fabrication and the polymer cytotoxicity.
Recently, advanced nanotechnology has undergone significant progression by using materials for the production and design of an optimal drug delivery system, since the physicochemical properties of polymers are used as an advantage to develop both liposomes and solid lipid NPs and to overcome their drawbacks during fabrication. These new structures are called polymeric hybrid lipid nanoparticles. Polymeric hybrid lipid nanoparticles contain three main structures, as follows: (1) a hydrophobic/hydrophilic polymeric core inserted inside liposomes or coated by a lipid monolayer; (2) a solid lipid core or liposomal phospholipid/cholesterol layer surrounded by a polymeric shell; (3) polymer materials incorporated inside solid lipid or liposomal phospholipid/cholesterol layers and then and an outer component consisting of a PEGylation.
Polymeric nanoparticles can be identified as an interaction of two opposite polymeric materials that form a cross-linked network and condensed core. This core can be further functionalized by a PEGylated layer or coated by a lipid monolayer. Hanafy et al. optimized hybrid polymeric lipid nanoparticles by using chitosan-oleic acid after blocking its free fatty acid. This strategy reduced the cytotoxicity that can be generated by oleic acid. Hybrid polymeric lipid nanoparticles can be co formulated in the shape of micelles by using polymer self-assembly, while drugs can either be attached during fabrication or inserted after fabrication. Recently, polymeric materials in the shape of hydrogel materials, mucoadhesive materials, and stimuli-responsive polymers have been developed. Polymeric nanoparticles are characterized by controlled drug release, stability inside cells, and easy and cost-effective formulation. However, the disadvantage of polymeric NPs is mostly associated with the type of organic solvent used during fabrication and the polymer cytotoxicity.
- Lung Cancer - Targeted therapies- Liposomes-
- Lipid nanoparticles- Polymeric nanoparticles
Dear author, the following contents are excerpts from your papers. They are editable.
(The entry will be online immediately after you submit it. The entry will better spread your research and findings and readers are able to access your paper directly through DOI number at the end of the entry. No worry about the format, we'll correct it after your submission)
1. Introduction
Lung cancer is the most frequently diagnosed cancer in the world and a common reason for cancer-related deaths [1]. For patients diagnosed with this type of cancer, the 5-year survival rate is approximately 17.8%[2] [2]. Lung cancer can be divided into three main subtypes according to microscopic evidence and histological profiles: non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), and lung carcinoid tumor, accounting for 85%, 10–15%, and less than 5% of cases, respectively[3] [3]. Among the three subtypes of lung cancer, NSCLC is the most common one diagnosed in non-smokers. It appears in women more than men, and it is more frequently discovered in younger people than other type of lung cancer[4] [4]. This type can be subdivided, according to World Health Organization (WHO), into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma[5] [5].
Adenocarcinoma is a popular type, representing around 40% of all total diagnostic cases. It usually occurs in smokers and nonsmokers[6] [6]. It arises from small airway epithelial cells that form the lining of the lung and alveolar cells: the mucus secreting cells[7] [7]. Furthermore, it grows slowly and can spread outside the lungs. Adenocarcinoma is characterized histologically by the presence of glandular/acinar growth, papillar differentiation, or a single-layer of wallpaper-like spread along the alveolar septum and bronchioles[8] [8]. Squamous-cell carcinoma is derived from the squamous cell type located at the airways of the epithelial cells. These cells line the bronchial tubes in the center of the lungs. This type is mostly associated with smoking tobacco, and it represents 30% of all lung cancer patients[9] [9]. It is histologically identified by the presence of keratinization or intercellular bridges.
Large cell carcinoma comprises 5–10% of lung cancer patients. It arises from the central region of the lungs, the area nearest to the lymph nodes, and the wall of the chest[10] [10]. It usually grows and spreads rapidly, which makes its treatment challenging. Large cell carcinoma, also called non–small cell cancer, has a poor prognosis[11] [11].
SCLC represents 25% of all invasive cancer types worldwide, and it is found exclusively in smokers[12] [12]. It originates from neuroendocrine cell precursors. Thus, it is attributed to endocrine and neurologic paraneoplastic syndromes (Eaton Lambert syndrome, inappropriate antidiuretic hormone secretion, and Cushing’s syndrome)[12] [12]. Moreover, it is characterized by its worse clinical course than that of NSCLC[13][14] [13,14]. Additionally, it can be resistant to both chemotherapy and radiotherapy courses[15][16] [15,16].
The last type of lung cancer is lung carcinoid tumor. It originates from neuroendocrine cells, which is are special cells located in the lungs. The growth of this type of cancer is typically very slow and it rarely spreads (see Figure 1)[17] [17].
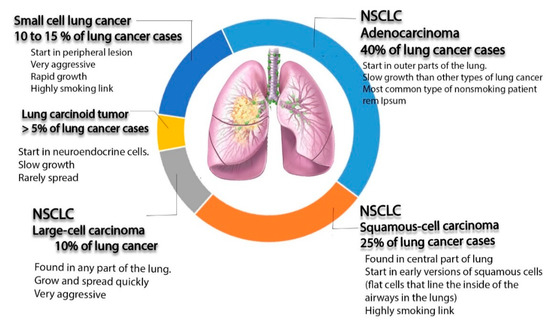
Figure 1. Schematic diagram illustrating different types of lung cancer (non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), and lung carcinoid tumors, as well as non-small cell lung cancer).
Here, we try to highlight the development and application of nanoparticles in lung cancer treatment, mainly those made of liposomes, lipids, and polymer materials. Many nanoparticle-based therapies have been developed for the treatment of metastatic NSCLC, such as liposomes, polymeric nanoparticles (NPs), albumin NPs, Lipid NPs, inorganic NPs, and metal NPs. However, few have been translated successfully into clinical trials. In our current study, liposomes, solid lipid nanoparticles (SLNPs), polymeric nanoparticles (PNPs), and hybrid polymeric materials were studied, and they are summarized in Table 1, Table 2 and Table 3, while the successful nanoparticles that moved into clinical trials are outlined in Table 4.
2. Clinical Studies of Pulmonary Nanoparticles Can Be Finally Driven into the Industry Market
Clinical trials represent strategy-based scientific research that attempts to evaluate the influence of a new therapeutic molecule on human health outcomes. It includes several steps, as follows: Phase I—the study of new therapeutic molecules on a small group of volunteers; Phase II—monitoring and evaluation of safe drugs on a large group of volunteers; Phase III—the study of safe drugs in different groups of volunteers located in different regions and countries; and Phase IV—monitoring of safe drugs after obtaining approval for their use in a wide population over long time frame[18] [112]. During the clinical trial process, each drug is given a particular description according to its status, as summarized in Table 4. For instance, it is “recruiting” when several participants are invited to contribute to the study, while “active” status means the study is underway. On the other hand, when the investigation is finished, there is no need for any participants. The status of the drug is changed to “completed”. “Terminated” status means that the study has stopped and will not be started again[19] [113]. In the current review, we have described the use of several nanochemotherapeutic agents to treat lung cancer, and recently, they have reached clinical trial status. Irinotecan liposome injection has obtained a “recurring” status and is being investigated in comparison with topotecan in patients with small cell lung cancer after treatment with a platinum-based first-line therapy (Phase 3; 2018–2022 study). Many studies have completed the clinical study process, such as Cholesterol-Fus1 in non-small-cell lung cancer[20] [114] and the liposomal form of Lurtotecan as OSI-211 to treat recurrent small cell lung cancer[21] [115]. Stimulating Targeted Antigenic Responses To NSCLC (START) was a phase III trial of the MUC1-antigen-specific cancer immunotherapy tecemotide, following chemoradiotherapy for unresectable stage III NSCLC[22] [116] and a liposomal formed of Lurtotecan [7(4-methylpiperazinomethylene)-10,11-ethylenedioxy-20-(S)-camptothecin dihydrochloride] was combined with cisplatin to treat patients with advanced or metastatic solid tumors. A phase II trial of this combination showed that three of 25 patients with breast cancer and two of 23 patients with NSCLC had partial responses[23] [117].
Table 4. Clinical trial studies of lung cancer therapies using lipid nanoparticles.
| Study Title | Conditions | Status | Assigned Number |
|---|---|---|---|
| Study of Irinotecan Liposome Injection (ONIVYDE®) in Patients With Small Cell Lung Cancer | Small Cell Lung Cancer | Recruiting | NCT03088813 |
| Irinotecan Hydrochloride Liposome Injection (LY01610) For Small Cell Lung Cancer | Small Cell Lung Cancer | Recruiting | NCT04381910 |
| Paclitaxel Liposome for Squamous Non-Small-cell Lung Cancer Study (LIPUSU) | Squamous Non-small-cell Lung Cancer | Active, not recruiting | NCT04381910 |
| Phase I Study of IV DOTAP: Cholesterol-Fus1 in Non-Small-Cell Lung Cancer | Lung Cancer | Completed | NCT00059605 |
| BLP25 Liposome Vaccine and Bevacizumab After Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | Lung Cancer | Active, not recruiting | NCT00828009 |
| Efficacy and Safety Study of OSI-211 (Liposomal Lurtotecan) to Treat Recurrent Small Cell Lung Cancer | SCLC and Carcinoma, Small Cell | Completed | NCT00046787 |
| Study of Tecemotide (L-BLP25) in Participants With Stage III Unresectable Non-Small Cell Lung Cancer (NSCLC) Following Primary Chemoradiotherapy | Non-small Cell Lung Cancer | Completed | NCT00960115 |
| Liposomal Lurtotecan Plus Cisplatin in Treating Patients With Advanced or Metastatic Solid Tumors | Head and Neck Cancer, Lung Cancer, Ovarian Cancer | Completed | NCT00006036 |
| Study of Autologous CIK Cell Immunotherapy Combination With PD-1 Inhibitor and Chemotherapy in Advanced NSCLC | Non-small Cell Lung Cancer | Recruiting | NCT03987867 |
| A Study of FF-10850 Topotecan Liposome Injection in Advanced Solid Tumors | Advanced Solid Tumors | Recruiting | NCT04047251 |
| TUSC2-Nanoparticles and Erlotinib in Stage IV Lung Cancer | Lung Cancer | Active, not recruiting | NCT01455389 |
| Doxil Topotecan, Doublet Cancer Study | Small Cell Lung Cancer, Pancreatic Cancer, Head and Neck Cancer | Completed | NCT00252889 |
| TUSC2-nanoparticles (GPX-001) and Osimertinib in Patients With Stage IV Lung Cancer Who Progressed on Osimertinib Alone | Carcinoma, Non-Small-Cell Lung | Not yet recruiting | NCT04486833 |
| Intrathecal Pemetrexed for Recurrent Leptomeningeal Metastases From Non-Small Cell Lung Cancer | Leptomeningeal Metastases | Completed | NCT03101579 |
| Inhaled Doxorubicin in Treating Patients With Primary Lung Cancer or Lung Metastases | Lung Cancer, Metastatic Cancer | Completed | NCT00004930 |
| VX-710, Doxorubicin, and Vincristine for the Treatment of Patients With Recurrent Small Cell Lung Cancer | Lung Cancer | Terminated | NCT00003847 |
| Topotecan Hydrochloride and Doxorubicin Hydrochloride in Treating Patients With Relapsed or Refractory Small Cell Lung Cancer | Recurrent Small Cell Lung Carcinoma | Completed | NCT00856037 |
| Inhaled Doxorubicin in Treating Patients With Advanced Solid Tumors Affecting the Lungs | Lung Cancer, Malignant Mesothelioma, Metastatic Cancer | Completed | NCT00020124 |
| Effects of STM 434 Alone or in Combination With Liposomal Doxorubicin in Patients With Ovarian Cancer or Other Advanced Solid Tumors | Ovarian Cancer, Fallopian Tube Cancer, Endometrial Cancer, Solid Tumors | Completed | NCT02262455 |
| An Open-label, Phase I/IIa, Dose Escalating Study of 2B3-101 in Patients With Solid Tumors and Brain Metastases or Recurrent Malignant Glioma. | Brain Metastases, Lung Cancer Breast Cancer | Completed | NCT01386580 |
| Radiation Therapy Plus Combination Chemotherapy In Treating Patients With Limited Stage Small Cell Lung Cancer | Lung Cancer | Completed | NCT00003364 |
| A Phase II Study of Doxorubicin, Cyclophosphamide and Vindesine With Valproic Acid in Patients With Refractory or Relapsing Small Cell Lung Cancer After Platinum Derivatives and Etoposide | Small Cell Lung Carcinoma | Completed | NCT00759824 |
| Combination Chemotherapy Followed by Radiation Therapy in Patients With Small Cell Lung Cancer | Lung Cancer | Completed | NCT00002822 |
Data retrieved from the US National Institutes of Health website (http://clinicaltrials.gov/) on 21 August 2011[19] [113].
Reference (we'll rearrange the references after you submitted it)
- Cokkinides, V.; Albano, J.; Samuels, A.; Ward, M.; Thum, J. American Cancer Society: Cancer Facts and Figures; American Cancer Society: Atlanta, GA, USA, 2005.
- Wong, M.C.S.; Lao, X.Q.; Ho, K.; Goggins, V.B.; Tse, S.L.A. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci. Rep. 2017, 7, 14300.
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193.
- Smolle, E.; Pichler, M. Non-Smoking-Associated Lung Cancer: A distinct Entity in Terms of Tumor Biology, Patient Characteristics and Impact of Hereditary Cancer Predisposition. Cancers 2019, 11, 204.
- Petersen, I. The morphological and molecular diagnosis of lung cancer. Dtsch. Arztebl. Int. 2011, 108, 525–531.
- Couraud, S.; Zalcman, G.; Milleron, B.; Morin, F.; Souquet, P.J. Lung cancer in never smokers—A review. Eur. J. Cancer 2012, 48, 1299–1311.
- Noguchi, M.; Morikawa, A.; Kawasaki, M.; Matsuno, Y.; Yamada, T.; Hirohashi, S.; Kondo, H.; Shimosato, Y. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995, 75, 2844–2852.
- Moulton, J.E. Tumors in Domestic Animals; University of California Press: Berkeley, CA, USA, 1978; pp. 203–205.
- Kenfield, S.A.; Wei, E.K.; Stampfer, M.J.; Rosner, B.A.; Colditz, G.A. Comparison of aspects of smoking among the four histological types of lung cancer. Tob. Control. 2008, 17, 198–204.
- Brambilla, E.; Pugatch, B.; Geisinger, K.; Gal, A.; Sheppard, M.; Guinee, D. Large cell carcinoma, World Health Organization Classification of Tumours. Pathol. Genet. Tumours Lung Pleura Thymus Heart 2004, 10, 45–50.
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300.
- Curran, W.J., Jr. Therapy of limited stage small cell lung cancer. Cancer Treat. Res. 2001, 105, 229–252.
- Mulshine, J.L.; Treston, A.M.; Brown, P.H.; Birrer, M.J.; Shaw, G.L. Initiators and promoters of lung cancer. Chest 1993, 103, 4S–11S.
- Zöchbauer-Müller, S.; Pirker, R.; Huber, H. Treatment of small cell lung cancer patients. Ann. Oncol. 1999, 10, S83–S91.
- Tamura, T. New state of the art in small-cell lung cancer. Oncology (Williston Park NY) 2001, 15, 8–10.
- Lad, T.; Piantadosi, S.; Thomas, P.; Payne, D.; Ruckdeschel, J.; Giaccone, G. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994, 106, 320S–323S.
- Lassen, U.; Hansen, H.H. Surgery in limited stage small cell lung cancer. Cancer Treat. Rev. 1999, 25, 67–72.
- Fatma, S.E.; Maggy, S.; Ahmed, M.; Maged, E.; Magdy, M.; Ola, E.; Sara, B.; Nemany, A.H. Drug Delivery Systems from Bench to Clinical Trials. Glob. J. Nano 2018, 4, 555636.
- Data Retrieved from US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/help/glossary/recruitment-status (accessed on 21 August 2011).
- Lu, C.; Stewart, D.J.; Lee, J.J.; Ji, L.; Ramesh, R.; Jayachandran, G.; Nunez, M.I.; Wistuba, I.I.; Erasmus, J.J.; Hicks, M.E.; et al. Phase I clinical trial of systemically administered TUSC2(FUS1)-nanoparticles mediating functional gene transfer in humans. PLoS ONE 2012, 7, e34833.
- Duffaud, F.; Borner, M.; Chollet, P.; Vermorken, J.B.; Bloch, J.; Degardin, M.; Rolland, F.; Dittrich, C.; Baron, B.; Lacombe, D.; et al. EORTC-New Drug Development Group/New Drug Development Program. Phase II study of OSI-211 (liposomal lurtotecan) in patients with metastatic or loco-regional recurrent squamous cell carcinoma of the head and neck. An EORTC New Drug Development Group study. Eur. J. Cancer 2004, 40, 2748–2752.
- Mitchell, P.; Thatcher, N.; Socinski, M.A.; Wasilewska-Tesluk, E.; Horwood, K.; Szczesna, A.; Martín, C.; Ragulin, Y.; Zukin, M.; Helwig, C.; et al. Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: Updated overall survival and biomarker analyses. Ann. Oncol. 2015, 26, 1134–1142.
- MacKenzie, M.J.; Hirte, H.W.; Siu, L.L.; Gelmon, K.; Ptaszynski, M.; Fisher, B.; Eisenhauer, E. A phase I study of OSI-211 and cisplatin as intravenous infusions given on days 1, 2 and 3 every 3 weeks in patients with solid cancers. Ann. Oncol. 2004, 15, 665–670.
