You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Shi Chen and Version 3 by Lindsay Dong.
Silicosis, characterized by irreversible pulmonary fibrosis, remains a major global public health problem. CNowadays, cumulative studies are focusing on elucidating the pathogenesis of silicosis in order to identify preventive or therapeutic antifibrotic agents. However, the existing research on the mechanism of silica-dust-induced pulmonary fibrosis is only the tip of the iceberg and lags far behind clinical needs. Idiopathic pulmonary fibrosis (IPF), as a pulmonary fibrosis disease, also has the same problem.
- silicosis
- IPF
- pulmonary fibrosis
- treatment drugs
1. Introduction
The prevalence of crystalline silicon dioxide dust is widespread in many areas [1]. As a result of the continuous inhalation of silica particles, many workers develop silicosis, an irreversible and incurable disease [2]. Silicosis is a chronic interstitial lung disease characterized by fibrosis, inflammation, and destruction of the pulmonary structures. It causes pulmonary hypertension, progressive dyspnoea and death from respiratory insufficiency [3]. Many measures have been taken in recent decades to protect workers in the workplace, but millions of workers still suffer from silicosis [4].
Silicosis is a severe concern in construction and mining workers, particularly young workers, who are exposed to quartz conglomerates during sandblasting, bolting, cutting, shaping, and installing kitchen countertops [5][6][7][5,6,7]. Recent studies have also indicated that exposure to nanosilica can cause inflammation and fibrosis in the lungs. The risk of nanosilica exposure in this emerging industry is noteworthy, despite the lack of reported cases [8]. The increasing number of silicosis cases worldwide presents new challenges for prevention in many countries [4]. The present circumstance underscores the significance of exercising caution in authorizing development of emerging industries and implementing early identification and control measures from a public health perspective. Furthermore, it is crucial to accelerate the discovery of remedies for silicosis from a clinical treatment standpoint.
The pathogenesis of silicosis is not fully understood, and the disease is complex [9][12]. Although further research is required to clarify the role of intricate signaling pathways [10][13], multiple pathways are thought to be involved in the development of silicosis (Figure 1). Silica-induced lung injury is characterized by various mechanisms, including direct cytotoxic effects on macrophages, activation of macrophage surface receptors, lysosomal rupture, reactive oxygen species (ROS) production, inflammasome activation, cytokine and chemokine production, apoptosis/softening, and lung fibrosis [11][14].
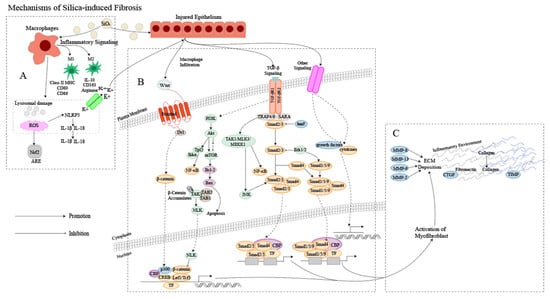

Figure 1. Mechanism of silica-induced fibrosis. (A) Alveolar macrophages (AMs) engulf silica dust, causing them to turn into dust cells. Subsequently, AMs may synergize with alveolar epithelial cells (AECs) to release a large amount of ROS to participate in oxidative stress reactions, activate NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammatory bodies through lysosomal damage and potassium outflux, and activate the release of inflammatory mediator interleukin (IL) -1β, IL-18 and other cytokines inducing epithelial–mesenchymal transition (EMT). Meanwhile, AMs can polarize into M1 and M2 types, playing a role in promoting inflammation, fibrosis, and antigen presentation, increasing the proliferation of lung fibroblasts and collagen synthesis and secretion, and promoting the formation of fibrosis through apoptosis and autophagy. (B) Ongoing damage and damage to lung cells by silica lead to pathological overdeposition of extracellular matrix (ECM) proteins accompanied by upregulation of myofibroblast activity, resulting in a chronic inflammatory environment of macrophage and immune cell infiltration. In this cellular environment, cytokines and growth factors are released in large quantities, activating many signaling cascades, including members of the transforming growth factor-beta (TGF-β) family and Wingless/Int (Wnt) 1, the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway and other pathways. (C) Fibroblasts then aggregate in the area of injury, and the combination of ECM degradation by MMPs and excessive collagen deposition leads to granuloma formation and lung tissue remodeling.
2. Characteristics of Pulmonary Fibrosis
2.1. Characteristics of IPF
The origin of idiopathic pulmonary fibrosis (IPF), much like silicosis, remains unclear, with a convoluted pathogenesis likely involving multiple interconnected signaling pathways, including the TGF-β/Smad signaling pathway, Wnt/β-catenin signaling pathway, platelet-derived growth factor (PDGF) signaling pathway, PI3K/AKT signaling pathway and other signaling pathways [12][16]. IPF is thought to be a consequence of damage to the alveolar epithelium and abnormal wound healing, but it has also been shown that both genetic factors and environmental conditions can significantly contribute to the development of the disease [13][14][17,18]. The condition is characterized by subpleural basal fibrosis, honeycomb changes, and collagen and ECM deposition, which ultimately result in life-threatening structural changes in lung tissue and loss of pulmonary ventilation and diffusion function.
Alveolar epithelial damage, caused by external factors (infection, toxins, smoke) or internal factors (inflammation, oxidative stress, abnormal immune response), leads to the release of fibrogenic cytokines, including TGF and tumor necrosis factor (TNF), as well as growth factors such as PDGF and connective tissue growth factor (CTGF) [15][19]. Elevated levels of these fibrogenic cytokines and growth factors, both locally and systemically, stimulate the activation and proliferation of lung fibroblasts to some extent. Upon activation, fibroblasts differentiate into pulmonary myofibroblasts, which are responsible for the excessive production of ECM proteins in fibrotic lung tissue. These myofibroblasts also regulate the balance between MMP and tissue inhibitors of metalloproteinases (TIMPs), thereby facilitating the process of IPF [16][20].
2.2. The Relationship between Silicosis and IPF
2.2.1. Cause of Disease
The inhalation of free silica dust in the air is the main cause of silicosis. The emergence of silicosis is closely linked to the volume, structure and dimensions of the silica particles inhaled [17][21], whereas IPF is a particular type of interstitial pneumonia that is fibrosing, chronic and progressive, but for which the origin is yet to be determined [18][22].
Despite the fact that occupational exposure can induce IPF to some extent, a plethora of epidemiological investigations have demonstrated a significant link between smoking, chronic viral infections, and the genetics of IPF [18][19][20][22,23,24].
2.2.2. Pathogenesis
Extensive research has revealed that the development of silicosis fibrosis is not solely due to one factor, but rather a complex outcome resulting from various factors and links [21][25]. The primary pathogenic mechanisms of silicosis involve direct cytotoxic effects, the generation of ROS and reactive nitrogen radicals, the release of inflammatory chemokines, the initiation of fibrotic pathways and cell death [4]. The cellular molecule and gene transcription regulation fields are also being explored in relation to silicosis [22][26].
Cellular senescence, which includes molecular changes such as telomere shortening, is involved in the pathogenesis of various chronic diseases, including lung diseases [23][28]. Telomere shortening may be a common causative feature of the development of IPF and silicosis [24][25][29,30].
Silicosis and IPF are respiratory diseases that cause damage to the lungs. In response to the inflammatory process, fibroblasts proliferate and produce excessive collagen fibers, leading to the deposition of ECM lung tissue remodeling, ultimately resulting in impaired lung function [26][27][33,34]. Moreover, the two diseases share similarities in the upregulation of TGF-β and extracellular signal-regulated kinase (ERK) signaling pathways in cytokine and growth factor pathways, and a relationship with autophagy [28][35]. While the immediate causes of silicosis and IPF may differ, the overall mechanisms of the subsequent profibrotic reaction are comparable, being characterized by ECM deposition and fibroblast proliferation [29][36]. Therefore, potential therapeutic drugs for the treatment of silicosis may be sought from IPF.
2.2.3. Symptoms and Complications
Silicosis and IPF share certain symptoms, such as dyspnoea and loss of appetite. However, patients with silicosis may experience additional manifestations, such as chest pain, pulmonary dysfunction, low-grade fever, night sweats, and active shortness of breath, while IPF typically presents as cough and sputum production [30][37]. In some severe cases, patients with IPF may also exhibit general discomfort, including weakness and joint pain.
The development of silicosis can result in various complications, including tuberculosis, chronic obstructive pneumonia, and rheumatoid arthritis [31][38]. Similarly, individuals with IPF may experience pulmonary hypertension, acute exacerbation of pulmonary fibrosis, respiratory tract infections, acute coronary syndrome, and thromboembolic disease [32][39]. However, as the diseases advance, both silicosis and IPF can increase the likelihood of developing lung cancer [33][34][35][40,41,42].
