Peritoneal carcinomatosis is a challenging condition that affects many cancer patients, and conventional therapies have limited efficacy in treating it. However, recent advances in the field of immunotherapy have shown promise in improving treatment outcomes. One promising approach is immune checkpoint inhibitors, which block proteins that inhibit T-cell activity and promote an anti-tumor immune response. Another approach involves the use of CAR-T cells, which are genetically modified T cells engineered to recognize and target cancer cells expressing specific antigens. In addition, dendritic cells and vaccine-based therapeutics are also designed to stimulate the immune system to recognize and attack cancer cells.
- intraperitoneal immunotherapy
- peritoneal carcinomatosis
- ascites
1. Introduction
2. Peritoneal Carcinomatosis
2.1. Peritoneum and Peritoneal Carcinomatosis
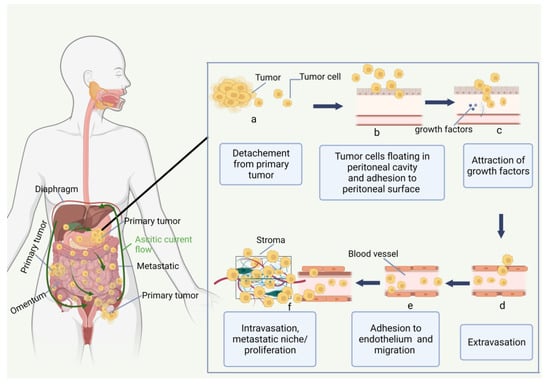
2.2. Immune Environment of Peritoneal Carcinomatosis
The innate (neutrophils, macrophages, dendritic cells, and natural killer cells) and the adaptive (B and T lymphocytes) immune systems can recognize and destroy tumor cells. However, cancer cells gain the ability to evade immune surveillance by targeting or manipulating the immune system. Since lymph nodes and the greater omentum both include immune cells such as macrophages and lymphocytes, immune cell activation may be a potential PC treatment strategy [22][16]. The peritoneal cavity has immunologically competent cells, such as 45% of monocytes/macrophages (CD68+), 45% of T-lymphocytes (CD2+), 8% of NK-cells (natural killer cells), and 2% of dendritic cells, as well as A substantial proportion of CD4+ (92%) and CD8+ (73%). Approximately 49% of the cells in the peritoneum were positive for class II major histocompatibility complex antigens [23][26]. In contrast to CD45RO-naive T-lymphocytes, CD45RO+ T-lymphocytes have already differentiated into memory and effector T cells. In contrast to peripheral blood cells, which have a predominance of CD8+ T-lymphocytes, healthy individuals have an inverted CD4+/CD8+ T-lymphocyte ratio. Innate immunity is activated by mesenchymal precursors of the peritoneum. Interleukin-1 (IL-1), interleukin-6 (IL-6), prostaglandin E2, granulocyte stimulating factor (GCSF), granulocyte monocyte colony-stimulating factor (GM-CSF), monocyte colony-stimulating factor (MCSF), and vascular epithelial growth factor (VEGF) are all pro-inflammatory mediators that are released by mesenchymal cells. Figure 2 shows the cell ascites tumor microenvironment (TME).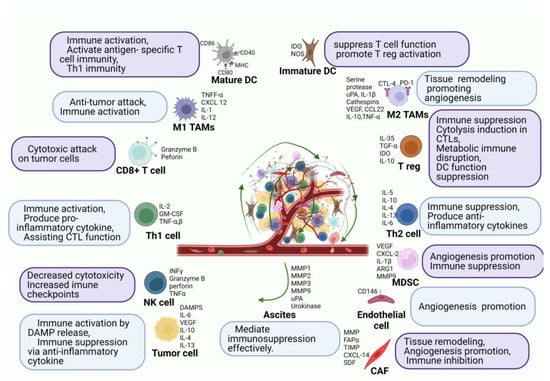
3. Immune Checkpoint Inhibitors
Patients with a wide variety of cancers benefit from antibodies that target immunological checkpoints, such as cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), PD-1, and PD-L1 [30][37]. In 2011, for the first time, the FDA approved ipilimumab as an immune checkpoint inhibitor (ICI) for the treatment of metastatic melanoma [31][38]. ICI has shown excellent and, most importantly, long-lasting responses in advanced tumor patients, unlike targeted treatment and chemotherapy. It is known that factors such as microsatellite instability (MSI-H), tumor mutation burden (TMB), and PD-L1 expression can be used to predict the therapeutic response of an ICI [32][41]. However, there is still insufficient evidence on whether these factors have a therapeutic effect in peritoneal metastasis. MSI-H cancers occur in gastrointestinal (colorectal, gastric, hepato-biliary) and endometrial malignancies and are caused by germline mutations in one of the DNA mismatch repair genes or somatic promoter hypermethylation of MLH. A high tumor mutation load boosts immunogenicity and ICI sensitivity [33][42]. After establishing a genetic signature-based predictive biomarker for systemic therapy (pembrolizumab, anti-PD-1 ICI across many tumor types), the FDA awarded its first tissue-agnostic clearance for deficient mismatch repair (dMMR)/MSI-H malignancies [34][43]. The FDA approval of pembrolizumab in dMMR/MSI-H was based on the KEYNOTE-158 study. The study evaluated the efficacy of pembrolizumab in patients with advanced solid tumors that had progressed on standard therapy. They found that pembrolizumab showed promising results in patients with dMMR/MSI-H solid tumors, with an overall response rate of 34.3% and a median duration of response of 24.4 months. The study suggests that pembrolizumab may be an effective treatment option for patients with dMMR/MSI-H solid tumors, including some pancreatic cancers [35][44]. In research utilizing a newly produced highly metastatic clone of murine gastric cancer cells, YTN16P, it was shown that infusion of PD-1 mAb through the intravenous or intraperitoneal route lowered the rate of metastasis development on the mesenteric surface by 30–40% as a monotherapy [36][46]. Additionally, previous studies utilizing colon [37][38][40,47] or ovarian cancer cells [39][40][41][48,49,50] have shown that anti-PD-1 mAb may partially, but not fully, inhibit the development of PM in immunocompetent animals. Mouse models established using YTN16 and LmcMF are resistant to ICI treatment because CXCL12 derived from CAFs recruit M2 macrophages which secrete various cytokines, such as VEGF, IL-10, amphiregulin, and MMP-1 [42][51]. These cytokines exhaust CD8+ cells, either directly or indirectly. Furthermore, infiltration of CD8+ cells is inhibited due to the high intertumoral pressure associated with tumor fibrosis induced by CAFs. Although these models are resistant to ICI therapy, anti-CAF treatment recovered the therapeutic efficacy of the ICI [43][52].4. Monoclonal Antibodies
4.1. MOC31PE Immunotoxin
The monoclonal antibody called MOC31PE is derived from the Pseudomonas exotoxin A (PE) and targets the epithelial cell adhesion molecule (EpCAM), a transmembrane glycoprotein that is significantly overexpressed in cancerous tissue, including HGSOC, and is expressed at small levels in normal tissue [44][45][59,60]. After attaching to the EpCAM-expressing surface of cancer cells, MOC31PE kills cells by deactivating crucial cellular functions. Additionally, MOC31PE has a competitive edge over earlier anti-EpCAM antibody-based treatments due to its “simpler” mode of action, requiring just binding to EpCAM-expressing cancer cells before directly promoting cancer cell death through toxin release inside the target cells [46][47][61,62]. The therapeutic efficacy of chemotherapy-resistant cancer cells can be enhanced by MOC31PE. Recently, it was shown that patients with metastatic carcinomas who express EpCAM showed good tolerance to systemic doses of MOC31PE [48][63]. The ImmunoPeCa experiment (NCT02219893), a phase 1 dose-escalation study carried out in 2017 [49][65], examined patients with peritoneal metastasis from colorectal cancer (CRC) after demonstrating anti-cancer efficacy in preclinical testing [46][50][51][61,64,66]. The MOC31PE immunotoxin was given intraperitoneally the day following surgery to 21 patients who had CRS/HIPEC for PC from CRC at four distinct dose levels. The medicine was found to be safe and well-tolerated, with no evidence of dose-limiting harm. Even though MOC31PE was not absorbed into the body very much, the levels in the peritoneal fluid were thought to be cytotoxic. Neutralizing antibodies were produced by all patients.4.2. Catumaxomab
Catumaxomab is a rat-murine bispecific and trifunctional antibody that targets EpCAM and can have a long-lasting immunization effect [52][53][70,71]. In 2009, catumaxomab was approved in Europe as the first drug for malignant ascites linked to PC [54][55][72,73]. This bispecific monoclonal antibody can target immune systems and has a safe profile in clinical trials when administered intravenously (IP). Catumaxomab’s fragment-crystallizable (Fc) domain activates Fc-receptor types I, IIa, and III on NK cells, CD3+ T-cells, and EpCAM receptors, which are the substance’s two antigen-binding sites that it particularly targets. As a result of this mechanism, pro-apoptotic cytokines including IL-2, IL-12, and TNF phagocytose the targeted tumor cells, leading to cell death [56][57][58][74,75,76]. Many ovarian cancer patients may already have peritoneal metastases at the time of their diagnosis, and the presence of a significant amount of malignant ascites accelerates the disease’s development and distention. In a trial by Burges et al., catumaxomab was used to treat ascites in 23 ovarian cancer patients who had resistant to conventional treatment. Production of ascites was significantly reduced during catumaxomab therapy in response to increasing dosages. Twenty-eight days after the last infusion, only one of twenty-three patients who received treatment needed a paracentesis, which is still nearly 2 weeks longer than is usually necessary [59][60][82,83]. In a multicenter trial conducted by Wimberger et al., 258 patients with ovarian and non-gynecologic malignancies were randomly assigned to treatment and control groups to determine the effect of catumaxomab therapy on life quality. Patient surveys were used to determine the results. Compared to paracentesis alone, treatment with catumaxomab with paracentesis considerably delayed the period until the quality of life deteriorated [61][84]. The therapy of malignant ascites from EpCAM+ tumors was evaluated in a randomized, multicenter study by Heiss et al. Catumaxomab significantly enhanced median puncture-free survival and time to next therapeutic intervention in the experimental group, as well as overall survival, among 258 patients with gastric cancer in this phase II/III clinical trial [57][75].5. Cancer Vaccines for Peritoneal Metastasis
Therapeutic vaccines against cancer are a further immunotherapy strategy that has attracted substantial recent advancements in the intraperitoneal developments of PC. Malignant ascites have a bad prognosis and are a significant barrier to the immune system responding to vaccines. To combat this, vaccines are currently being developed and modified to specifically target ascites to enhance the quality of life for PM patients.
Cellular, viral vector, and molecular (peptide, DNA, or RNA) are the three main platforms for cancer vaccines [62][86]. Allogeneic tumor cell lines or autologous patient-derived tumor cells are used to create cellular vaccines [63][87]. Due to their functions as tumor antigen consumers, processors, and presenters, dendritic cells (DCs) are employed to create cellular cancer vaccines. Oncolytic viral vaccinations have been genetically altered to target and kill tumor cells [64][88]. In addition to their oncolytic effects, viral vectors also stimulate tumor-specific immune responses by providing tumor antigens through more typical T-cell priming procedures [65][89]. On the cell surface, major histocompatibility complex (MHC) peptides expression can be detected by T-cells [66][90]. For the creation of peptide-based cancer vaccines, it is important to know how peptides and T cell receptors interact with MHC. Enzymes break down short peptides, which are typically nine amino acid residues long, and immediately connect to MHC molecules, perhaps generating tolerance [67][91]. Longer peptides, typically 30 mer, are taken in by antigen-presenting cells (APCs), processed for MHC presentation, and result in memory CD4+ and CD8+ T cell immunological responses, which may make APCs more immunogenic [67][91]. DNA vaccines, often known as “naked DNA”, are closed circular DNA plasmids that encode TAAs and immunomodulatory substances intending to induce tumor-specific immune responses [68][92]. Despite being straightforward, secure, and quick to create, naked DNA vaccines are ineffective against target tumor cells due to low rates of transfection. mRNA vaccines, which are produced in vitro, encode an antigen or antigens, and following internalization, they express proteins that cause an immune reaction. mRNA vaccines may convey a large number of antigens and co-stimulatory signals without running the risk of infection or insertional mutagenesis, and their manufacture is rapid and affordable. However, the delivery effectiveness and stability are issues for mRNA vaccines [68][92].
Targeting ascites in PC has been accomplished by combining DCs with cytokine-induced killer cells (CIKs), which are cytotoxic T lymphocytes with a CD3+ CD56+ phenotype. The choice of CIKs was made based on three important criteria: they exhibit low cytotoxicity toward normal cells, no negative impact on hematopoiesis in the bone marrow, and resistance to Fas ligand-induced apoptosis. The effects of the combined treatment of DCs and CIKs include an increase in cytotoxic T cells in ascites that are driven by TNF and IFN and a reduction in immunosuppressive Tregs [69][93]. Similar to CAR-T cells, the method by which cancer vaccines are administered plays an important role in their dissemination. Natural killer cells (NKs) and dendritic cells (DCs) working together to fight tumors have been proven to be successful. Geller et al. demonstrated that IP- injection of IL-2-activated NK cells enhanced antitumor effects in an ovarian cancer mouse model xenograft, in contrast to systemic distribution [70][94].
Many cycles of patient-derived type I CD4+ T helper cells (Th1) provided by IP together with the cytokines IL-2 and IFN were shown to improve the anti-tumor activity of autologous CD8+ T cells against the tumor-specific glycoform of MUC1. This was reported by Dobrzanski et al. [71][72][98,99]. In a peritoneal metastatic colon cancer murine model, Alkayyal et al. further emphasized the relevance of combining pro-inflammatory cytokine IL-12 with an oncolytic virus (Maraba MG1) for reducing tumor burden in a CT26 colon cancer model. When MG1-IL12-ICV was IP-administered to these animals, it significantly decreased tumor development, created resistance to CT26 cell reinoculation, and improved survival. Regarding the mechanism, IL-12 was effective in enticing NK cells to the tumor location for annihilation. When paired with MG1 viral proteins, these activated NK cells generated IFN, which stimulated DCs and aided in the attraction of more NK cells [73][100].
