Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Fanny Huang and Version 1 by Brandon Lucke-Wold.
Focused ultrasound technology provides a method for overcoming the blood–brain and blood–tumor barriers through ultrasound frequency to transiently permeabilize or disrupt these barriers. Concomitant delivery of therapeutics has allowed for previously impermeable agents to reach the tumor microenvironment.
- microbubble-enhanced focused ultrasound
- chemotherapy
- focus ultrasound
- brain tumors
1. Introduction
Ultrasound has been a vital imaging modality since the 1970s [1]. Although ultrasound has primarily been used for rapid and cost-effective visualization of intra-abdominal, pelvic, and cardiac anatomy, and sparingly used in neurology and neurosurgery (i.e., transcranial and carotid Doppler) [2]. Its ability to transmit focused energy into soft tissues and utilize scattered energy to create pre- and post-operating images for interventional care has enhanced patient care and outcomes [3,4][3][4]. For example, ultrasound can assist surgical guidance in the operating room to allow the examination of various neurological pathways and diseases. Image formation relies on the transmission of ultrasound propagating through tissue at a rate of approximately 1.5 mm/µs, from which the penetration depth and outline of brain tumors can be calculated [4].
Aside from imaging, focus ultrasound (FUS) can be used to ablate tissue. FUS has regulatory approval with approved insurance reimbursement for neurologic diseases, such as benign essential tremor (ET) and Parkinson’s Disease (PD) [5,6,7][5][6][7]. For ET, Iorio-Morin et al. treated 10 patients with a unilateral FUS thalamotomy. Their results indicated that a majority of these patients experienced an improvement in their tremors with mild adverse effects of dysphagia following FUS treatment [5]. Similarly, Elias et al. reported hand-tremor improved after FUS thalamotomy with the most common adverse event being gait disturbance [7,8][7][8]. Mitigating these possible adverse effects could prove vital in the progression and increased use of this therapy in patients with benign ET. The benefit of thalamotomy for medication-refractory ET lasts up to 3 years with no progressive or delayed adverse effects [9]. Due to the progress of these clinical trials, the American Society of Stereotactic and Functional Neurosurgery (ASSFN) has published a set of best-practice statements to guide use of MR-guided FUS in treatment-refractory ET [10].
Similarly, for patients diagnosed with PD, randomized trials have shown that the use of FUS-mediated subthalamotomy can improve dyskinesias [6,11,12][6][11][12]. In patients for whom deep brain stimulation may be contraindicated, this serves as a less invasive alternative treatment route [13,14][13][14].
In addition to directly being used as an ablative therapy, when combined with microbubbles, FUS can be used to transiently disrupt the blood–brain barrier (BBB) and brain–tumor barrier (BTB) and potentially provide patients an enhanced administration of treatments (Figure 1) [15,16,17][15][16][17]. Microbubbles are nano to micron sized, gas-filled particles that volumetrically oscillate when exposed to ultrasound. The gas is commonly encapsulated in a lipid shell, though protein and polymer shells have also been explored [18]. The anatomy of a healthy BBB involves many cells that regulate the transport of osmotically active molecules between the brain parenchyma and surrounding vasculature [19]. Brain capillaries are surrounded by pericytes, microglia, astrocytes, and junctional complexes (tight, adherens, gap) that mediate permeability, monitor immune cell infiltration, and regulate flow of cerebrospinal fluid (CSF) [20]. Selective transmembrane proteins, such as P-glycoproteins, and efflux transporters can use ATP to actively export intraparenchymal solutes. In contrast, the BTB is created by primary or metastatic cancer cells initiating angiogenesis to form a unique vascular network directly surrounding the tumor. Much of this neurovasculature is leaky as tumor angiogenesis fails to faithfully recreate BBB features, such as tight junctions [21]. Furthermore, Sprowls et al. reported that tumors can disrupt the BBB through down-regulation of junction proteins such as Mfsd2a [19]. Tumors also induce a pro-inflammatory state which promotes microglial secretion of VEGF, inducing a vasogenic edema [19,22][19][22]. These studies highlight the complex interplay between tumor tissue and its vascular supply which can confound the effective delivery of therapeutics.
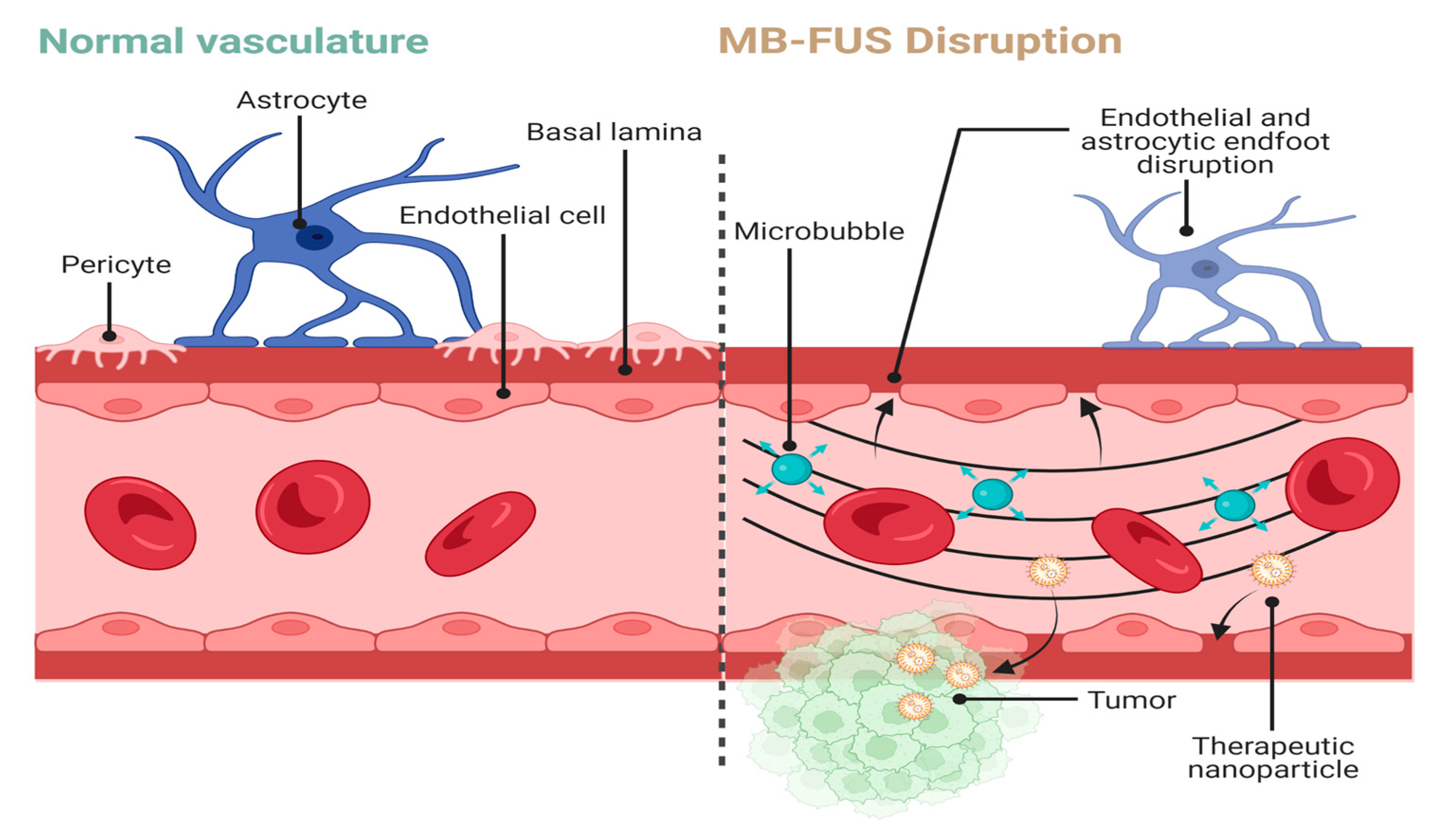
Figure 1. MB-FUS-mediated BBB disruption for therapy delivery. Normal vasculature consists of astrocytic endfeet surrounding endothelial cells linked by junctional complexes. MB-FUS disrupts these interactions to allow for passage of therapeutics into the tumor microenvironment. Arrows represent areas of disruption in the BBB. Created with BioRender.com.
The BBB and BTB are clinically important as they limit the passage of therapeutics into the tumor microenvironment (TME) [23,24,25,26][23][24][25][26]. For example, Ranjan et al. detailed stem cell assay-guided chemotherapy for patient-specific glioma subtypes, but these chemotherapeutic regimens are limited by the impermeability of the BBB [27]. Significant advances have been made in the preclinical delivery of glioma therapeutics using focused ultrasound (FUS). Currently, the DNA alkylator, Temozolomide (TMZ), is one of the few chemotherapeutics which has the capability of bypassing the BBB because of its small size and lipophilicity [28]. It is now the gold standard for adjuvant treatment in several brain tumors [29]. However, even with its ability to bypass the BBB, effective delivery of TMZ to the TME is further impeded by the heterogenous blood supply to the tumor bulk. In particular, the increased interstitial fluid pressure (IFP) between the tumor cells and their blood supply leads to heterogenous concentrations of chemotherapy within the tumor bulk [30,31][30][31]. With these barriers and the consequent poor bioavailability of TMZ in the TME, TMZ must be delivered more frequently, increasing the risk of adverse side effects. However, microbubble-enhanced focused ultrasound (MB-FUS) has promise to solve this bioavailability issue as molecules, such as doxorubicin and trastuzumab, have been reported to have increased uptake patterns when MB-FUS was utilized to disrupt the BBB/BTB barriers [19,32,33][19][32][33].
2. Mechanisms behind MB-FUS-Mediated BBB Disruption and Permeabilization
FUS is a powerful tool for modifying the BBB and BTB. FUS traditionally has two modes, high-intensity and low-intensity. High-intensity FUS can quickly raise the temperature of tissues allowing for precise and targeted thermal ablation [34]. Coagulative necrosis is often the result of thermal ablation [35]. Low-intensity FUS, in combination with microbubbles, can safely disrupt the BBB in a manner that does not cause significant irreversible damage to peripheral tissues and cells [34].
2.1. Microbubble Composition and Characteristics
Low intensity FUS-mediated disruption of the BBB requires exogenous microbubble administration for safe, transient permeabilization. Exogenous microbubbles are comprised of gas encapsulated by protein, lipids, or polymers with commercially available microbubbles ranging from 1 to 10 m [36,37][36][37]. Their composition can vary greatly in ratios of polymers, proteins, lipids, and gases [34,35][34][35]. They are commonly administered as ultrasound contrast agents [35].
Microbubbles are an essential component of FUS-mediated BBB disruption. They convert ultrasound (US) energy, effectively reducing the amount of required US energy to induce BBB disruption compared to FUS alone [38]. Microbubbles are highly compressible and cavitate under ultrasound [39]. Cavitation is the phenomena whereby microbubbles oscillate and collapse due to harmonics [35].
Two types of cavitation exist, inertial and stable. Inertial cavitation is often associated with higher ultrasound intensities. At these intensities, microbubbles expand and collapse, with the collapse being dominated by the inertia of the surrounding fluid [34]. When they collapse, they can produce jetting, free radicals, and shock waves [34,40][34][40]. Consequently, nearby blood vessels can be damaged resulting in petechiae, ischemia, or hemorrhage [34]. Stable cavitation typically occurs when microbubbles oscillate with smaller amplitudes within the ultrasound field. These oscillations mediate the transfer of energy to the local fluid environment through microstreaming around the bubble and shear stress imparted on nearby vessel walls [34,40][34][40]. Stable cavitation, induced by low and high frequencies, is the driver of contemporary BBB disruption investigations [35]. It can temporarily disrupt the junctional complexes of the endothelial cells within the BBB for up to 24 h depending on the parameters used [41,42,43][41][42][43]. One means by which this occurs is the deformation of cellular membranes and subsequent activation of mechanosensitive ion channels which increase membrane permeability [41].
2.2. Post Low-Intensity FUS Cellular and Biochemical Changes
Low-intensity FUS stimulates a variety of intracellular biochemical responses apart from the physical effects of cavitation. These include, but are not limited to, reductions in gap and tight junction proteins [35], potentiation of transcytosis [29], and downregulation of P-glycoprotein [36]. FUS is also hypothesized to induce changes to BBB permeabilization regulatory pathways, such as the phosphatidylinositol 3-kinase/Akt pathway [35]. Cellular adhesion molecules (CAMs) are another cellular component of the BBB which have been shown to aid in the transmigration of CD4+ T-cells across the BBB via an interaction with T-cell associated lymphocyte function-associated antigen 1 (LFA-1) [44]. Interestingly, FUS upregulates intercellular adhesion molecule 1 (ICAM1) for 24 h [45]. This transient upregulation may play a role in the FUS-mediated immunostimulation discussed in later sections.
3. MB-FUS as a Delivery Method (Clinical)
The continuous non-fenestrated capillaries of the BBB prevents the entry of neurotoxic molecules [87][46]. Although this is advantageous in reducing pathogens and preserving homeostasis, it is a barrier to the delivery of pharmaceutical treatments [88][47]. With each successive administration of MB-FUS, the force needed to disrupt the tight junctions of the capillaries reduces. This way, the BBB is more efficiently opened for the improved uptake of drugs to the brain. Various systems exist which serve to deliver MB-FUS to disrupt the BBB and improve the delivery of drugs [89][48].
3.1. Focused Ultrasound Commercial Systems
There are different systems of delivering FUS to increase the permeability of the BBB, including Exablate Neuro, Sonocloud-1/9, and NaviFUS (Figure 2). As described by Chen et al., Sonocloud-1 by CarThera requires a transcranial implant for the delivery of ultrasound to the BBB. While implanted, this device delivers a 25,000-cycle pulse at 1 Hz for 4 min. CarThera has also created an upgraded Sonocloud-9 system which uses nine transcranial implants for the ability to deliver more localized US intensity. With Exablate, a hemi-spherical transducer for the delivery of the ultrasound is placed over the top of the skull with 1024 transducer elements delivering a frequency of 620–720 kHz [90][49]. The Exablate requires the concurrent use of MRI with FUS for imaging [79][50]. MRI imaging is used to correctly localize the region to target for therapy. Microbubbles are also delivered intravenously for the improved permeability of the BBB [91][51]. NaviFUS allows personalized modulation of ultrasound intensity and amplitude for the individual patient. It also contains neuronavigation that helps target ultrasound to the tumor [79][50].
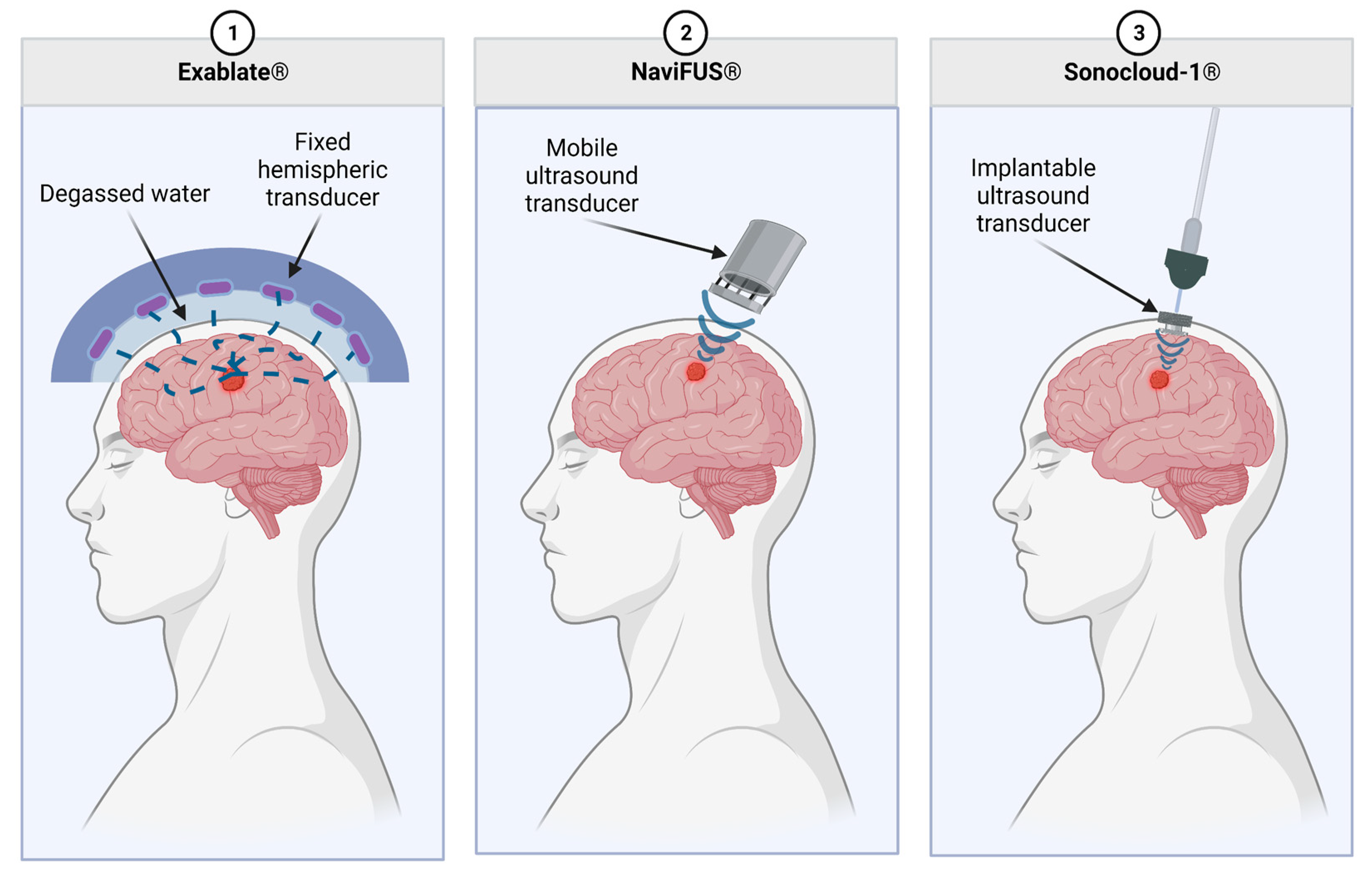
Figure 2. Commercially available MB-FUS modalities. Exablate uses a fixed, hemispheric transducer which is separated from the patient by a layer of degassed water. The NaviFUS is a portable ultrasound transducer which allows for slightly more flexibility in treating various tumor localizations. Lastly, the Sonocloud-1 has an implantable transducer which connects to external equipment. This allows for a highly reproducible dose to be delivered to the TME over multiple treatments.
These various methods each have their advantages and disadvantages. NaviFUS benefits from less invasiveness, user-friendliness, and minimal extra equipment, such as an MRI [88][47]. Sonocloud-1/9 has the benefit of ease of repeated treatments. Exablate theoretically has more control over the intensity and localization of FUS given the increased number of transducers and MRI guidance.
3.2. Chemotherapy Delivery via MB-FUS
Idbaih et al. used MB-FUS to deliver IV carboplatin to improve drug delivery for glioblastoma patients. This was accomplished with the SonoCloud-1 system where the transducer was placed via a burr hole through the skull bone overlying the tumor area at the external face of the dura mater [92,93][52][53]. As this was an escalating ultrasound experiment, the ultrasound pressure was gradually escalated from 0.41 MPa to 1.15 MPa at seven different levels [92][52]. The investigators of this experiment ensured at least three subjects were treated with each of the seven levels of the ultrasound pressure [92][52]. Patients recruited had recurrent de novo GBM after being treated with standard of care (radiation with adjuvant TMZ). Patients received IV 0.1 mL/kg SonoVue Microbubbles followed by pulsed ultrasound frequency of either 0.5 or 1 Hz through the SonoCloud-1 implant for 150–270 s. Immediately following MB-FUS, patients received an IV carboplatin (AUC4, AUC5, or AUC6) infusion for 60–90 min. Confirmation of BBB opening was done via scheduled post-treatment MRI. This treatment regimen occurred every four weeks for a maximum of six treatments [92][52]. While carboplatin is used as a third-line treatment for GBM, it has been shown to reduce tumor size in glioblastoma [92,94,95,96,97,98,99,100,101][52][54][55][56][57][58][59][60][61]. Therefore, by increasing the permeability of the BBB through MB-FUS, they hypothesized intratumoral carboplatin concentration would increase [92][52].
For those who obtained at least a grade 2 opening of the BBB as quantified by post-treatment MRI, the progression free survival (PFS) was 4.11 months, and the overall survival (OS) was 12.94 months [92][52]. Among patients with insufficient BBB permeabilization, PFS was 2.74 months, and OS was 8.64 months [92][52]. This demonstrates a correlation between achieving an increased opening in the BBB with concomitant chemotherapy delivery and improved survival.
During this experiment, 67% of the adverse effects were graded as 1 or 2 according to the CTAE. The most common adverse effects were hematological disorders at 32% and fatigue at 19%. Dose limiting toxicities were not apparent during or after the course of the treatments. Among central nervous system (CNS) adverse effects, the most common were headaches (26%), cerebral edema (11%), and syncope (11%). Few patients presented with transient facial palsy which improved within two hours after corticosteroid treatment. The most severe adverse effect observed was grade 4 edemas in two patients (11%). In both cases, symptoms resolved within two hours after corticosteroid therapy. After weighing the risks and benefits of MB-FUS-mediated carboplatin delivery, there appears to be a therapeutic benefit. Further clinical trials will need to be conducted with other chemotherapeutics and varying MB-FUS settings.
Another phase I trial used the updated Sonocloud-9 system to deliver albumin-bound paclitaxel in patients with recurrent GBM [102][62]. Patients underwent MB-FUS every three weeks for up to six cycles with dose escalation of paclitaxel up to 260 mg/m2. Given that this was a phase I trial, the researchers were only able to comment on safety. The main severe adverse effect noted was self-resolving encephalopathy in one patient with several patients experiencing mild headache as the predominant side effect. A phase II trial is ongoing.
3.3. Immunotherapy Delivery via MB-FUS
Current trials are underway to examine the efficacy of pembrolizumab (Keytruda) with the use of Exablate for metastatic brain cancer. Pembrolizumab is shown on its own to improve clinical outcomes in patients with metastatic glioblastoma [103][63]. The treatment will be provided every 3 weeks with the use of Exablate preceding the infusion of pembrolizumab to target the BBB for improved uptake [104][64]. The primary outcome is a response of tumor burden compared to baseline as measured by MRI every three weeks for a total of six months.
3.4. Ongoing Trials
The Toronto group is focusing on ultrasound induced capability to obtain liquid biopsies in the BRAINFUL Trial [105][65]. From a treatment standpoint, several groups are looking into treatment for Parkinson’s disease, movement disorders, temporal lobe epilepsy, and neurodegenerative dementias. Exablate is also being investigated for diffuse intrinsic pontine gliomas and brain metastasis at high frequency with and without chemotherapy regimens. Initial safety studies have been demonstrated for glioma and FUS-mediated chemotherapy delivery [106,107][66][67]. Emerging innovation is being investigated in terms of anxiety, depression, and pain relief. At low intensity, focused ultrasound is being utilized for memory enhancement and stroke rehabilitation. From a more mechanistic standpoint, several groups are looking at BBB disruption and association with glymphatic clearance [108][68].
References
- Troxclair, L.; Smetherman, D.; Bluth, E.I. Shades of Gray: A History of the Development of Diagnostic Ultrasound in a Large Multispecialty Clinic. Ochsner. J. 2011, 11, 151–155.
- Deffieux, T.; Demené, C.; Tanter, M. Functional Ultrasound Imaging: A New Imaging Modality for Neuroscience. Neuroscience 2021, 474, 110–121.
- Sastry, R.; Bi, W.L.; Pieper, S.; Frisken, S.; Kapur, T.; Wells, W.; Golby, A.J. Applications of Ultrasound in the Resection of Brain Tumors. J. Neuroimaging 2017, 27, 5–15.
- Rabut, C.; Yoo, S.; Hurt, R.C.; Jin, Z.; Li, H.; Guo, H.; Ling, B.; Shapiro, M.G. Ultrasound Technologies for Imaging and Modulating Neural Activity. Neuron 2020, 108, 93–110.
- Iorio-Morin, C.; Yamamoto, K.; Sarica, C.; Zemmar, A.; Levesque, M.; Brisebois, S.; Germann, J.; Loh, A.; Boutet, A.; Elias, G.J.B.; et al. Bilateral Focused Ultrasound Thalamotomy for Essential Tremor (BEST-FUS Phase 2 Trial). Mov. Disord. 2021, 36, 2653–2662.
- Moosa, S.; Martínez-Fernández, R.; Elias, W.J.; Del Alamo, M.; Eisenberg, H.M.; Fishman, P.S. The Role of High-Intensity Focused Ultrasound as a Symptomatic Treatment for Parkinson’s Disease. Mov. Disord. 2019, 34, 1243–1251.
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2016, 375, 730–739.
- Elias, W.J.; Huss, D.; Voss, T.; Loomba, J.; Khaled, M.; Zadicario, E.; Frysinger, R.C.; Sperling, S.A.; Wylie, S.; Monteith, S.J.; et al. A Pilot Study of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2013, 369, 640–648.
- Halpern, C.H.; Santini, V.; Lipsman, N.; Lozano, A.M.; Schwartz, M.L.; Shah, B.B.; Elias, W.J.; Cosgrove, G.R.; Hayes, M.T.; McDannold, N.; et al. Three-Year Follow-up of Prospective Trial of Focused Ultrasound Thalamotomy for Essential Tremor. Neurology 2019, 93, e2284–e2293.
- Pouratian, N.; Baltuch, G.; Elias, W.J.; Gross, R. American Society for Stereotactic and Functional Neurosurgery Position Statement on Magnetic Resonance-Guided Focused Ultrasound for the Management of Essential Tremor. Neurosurgery 2020, 87, E126–E129.
- Sinai, A.; Nassar, M.; Sprecher, E.; Constantinescu, M.; Zaaroor, M.; Schlesinger, I. Focused Ultrasound Thalamotomy in Tremor Dominant Parkinson’s Disease: Long-Term Results. J. Park. Dis 2022, 12, 199–206.
- Abusrair, A.H.; Elsekaily, W.; Bohlega, S. Tremor in Parkinson’s Disease: From Pathophysiology to Advanced Therapies. Tremor Other Hyperkinet. Mov. 2022, 12, 29.
- Martínez-Fernández, R.; Máñez-Miró, J.U.; Rodríguez-Rojas, R.; del Álamo, M.; Shah, B.B.; Hernández-Fernández, F.; Pineda-Pardo, J.A.; Monje, M.H.G.; Fernández-Rodríguez, B.; Sperling, S.A.; et al. Randomized Trial of Focused Ultrasound Subthalamotomy for Parkinson’s Disease. N. Engl. J. Med. 2020, 383, 2501–2513.
- Bond, A.E.; Shah, B.B.; Huss, D.S.; Dallapiazza, R.F.; Warren, A.; Harrison, M.B.; Sperling, S.A.; Wang, X.-Q.; Gwinn, R.; Witt, J.; et al. Safety and Efficacy of Focused Ultrasound Thalamotomy for Patients With Medication-Refractory, Tremor-Dominant Parkinson Disease. JAMA Neurol. 2017, 74, 1412–1418.
- Konofagou, E.E.; Tung, Y.-S.; Choi, J.; Deffieux, T.; Baseri, B.; Vlachos, F. Ultrasound-Induced Blood-Brain Barrier Opening. Curr. Pharm. Biotechnol. 2012, 13, 1332–1345.
- Meng, Y.; Hynynen, K.; Lipsman, N. Applications of Focused Ultrasound in the Brain: From Thermoablation to Drug Delivery. Nat. Rev. Neurol. 2021, 17, 7–22.
- Meng, Y.; Pople, C.B.; Lea-Banks, H.; Abrahao, A.; Davidson, B.; Suppiah, S.; Vecchio, L.M.; Samuel, N.; Mahmud, F.; Hynynen, K.; et al. Safety and Efficacy of Focused Ultrasound Induced Blood-Brain Barrier Opening, an Integrative Review of Animal and Human Studies. J. Control. Release 2019, 309, 25–36.
- Stride, E.; Segers, T.; Lajoinie, G.; Cherkaoui, S.; Bettinger, T.; Versluis, M.; Borden, M. Microbubble Agents: New Directions. Ultrasound Med. Biol. 2020, 46, 1326–1343.
- Sprowls, S.A.; Arsiwala, T.A.; Bumgarner, J.R.; Shah, N.; Lateef, S.S.; Kielkowski, B.N.; Lockman, P.R. Improving CNS Delivery to Brain Metastases by Blood-Tumor Barrier Disruption. Trends Cancer 2019, 5, 495–505.
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional Proteins of the Blood-Brain Barrier: New Insights into Function and Dysfunction. Tissue Barriers 2016, 4, e1154641.
- Guyon, J.; Chapouly, C.; Andrique, L.; Bikfalvi, A.; Daubon, T. The Normal and Brain Tumor Vasculature: Morphological and Functional Characteristics and Therapeutic Targeting. Front. Physiol. 2021, 12, 622615.
- Esquenazi, Y.; Lo, V.P.; Lee, K. Critical Care Management of Cerebral Edema in Brain Tumors. J. Intensive Care Med. 2017, 32, 15–24.
- Harder, B.G.; Blomquist, M.R.; Wang, J.; Kim, A.J.; Woodworth, G.F.; Winkles, J.A.; Loftus, J.C.; Tran, N.L. Developments in Blood-Brain Barrier Penetrance and Drug Repurposing for Improved Treatment of Glioblastoma. Front. Oncol. 2018, 8, 462.
- Bhowmik, A.; Khan, R.; Ghosh, M.K. Blood Brain Barrier: A Challenge for Effectual Therapy of Brain Tumors. BioMed Res. Int. 2015, 2015, 320941.
- Mungur, R.; Zheng, J.; Wang, B.; Chen, X.; Zhan, R.; Tong, Y. Low-Intensity Focused Ultrasound Technique in Glioblastoma Multiforme Treatment. Front. Oncol. 2022, 12, 903059.
- Mehkri, Y.; Woodford, S.; Pierre, K.; Dagra, A.; Hernandez, J.; Reza Hosseini Siyanaki, M.; Azab, M.; Lucke-Wold, B. Focused Delivery of Chemotherapy to Augment Surgical Management of Brain Tumors. Curr. Oncol. 2022, 29, 8846–8861.
- Ranjan, T.; Sengupta, S.; Glantz, M.J.; Green, R.M.; Yu, A.; Aregawi, D.; Chaudhary, R.; Chen, R.; Zuccarello, M.; Lu-Emerson, C.; et al. Cancer Stem Cell Assay-Guided Chemotherapy Improves Survival of Patients with Recurrent Glioblastoma in a Randomized Trial. CR Med. 2023, 4, 101025.
- Agarwala, S.S.; Kirkwood, J.M. Temozolomide, a Novel Alkylating Agent with Activity in the Central Nervous System, May Improve the Treatment of Advanced Metastatic Melanoma. Oncologist 2000, 5, 144–151.
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996.
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Östman, A. High Interstitial Fluid Pressure—An Obstacle in Cancer Therapy. Nat. Rev. Cancer 2004, 4, 806–813.
- Jain, R.K. Barriers to Drug Delivery in Solid Tumors. Sci. Am. 1994, 271, 58–65.
- Jin, Q.; Kang, S.-T.; Chang, Y.-C.; Zheng, H.; Yeh, C.-K. Inertial Cavitation Initiated by Polytetrafluoroethylene Nanoparticles under Pulsed Ultrasound Stimulation. Ultrason. Sonochem. 2016, 32, 1–7.
- Treat, L.H.; McDannold, N.; Zhang, Y.; Vykhodtseva, N.; Hynynen, K. Improved Anti-Tumor Effect of Liposomal Doxorubicin after Targeted Blood-Brain Barrier Disruption by MRI-Guided Focused Ultrasound in Rat Glioma. Ultrasound. Med. Biol. 2012, 38, 1716–1725.
- Bunevicius, A.; McDannold, N.J.; Golby, A.J. Focused Ultrasound Strategies for Brain Tumor Therapy. Oper. Neurosurg. 2020, 19, 9–18.
- Hersh, A.M.; Bhimreddy, M.; Weber-Levine, C.; Jiang, K.; Alomari, S.; Theodore, N.; Manbachi, A.; Tyler, B.M. Applications of Focused Ultrasound for the Treatment of Glioblastoma: A New Frontier. Cancers 2022, 14, 4920.
- Choi, J.J.; Feshitan, J.A.; Baseri, B.; Wang, S.; Tung, Y.-S.; Borden, M.A.; Konofagou, E.E. Microbubble-Size Dependence of Focused Ultrasound-Induced Blood-Brain Barrier Opening in Mice in Vivo. IEEE Trans. Biomed. Eng. 2010, 57, 145–154.
- Wu, S.-K.; Chu, P.-C.; Chai, W.-Y.; Kang, S.-T.; Tsai, C.-H.; Fan, C.-H.; Yeh, C.-K.; Liu, H.-L. Characterization of Different Microbubbles in Assisting Focused Ultrasound-Induced Blood-Brain Barrier Opening. Sci. Rep. 2017, 7, 46689.
- Burgess, A.; Hynynen, K. Drug Delivery across the Blood-Brain Barrier Using Focused Ultrasound. Expert. Opin. Drug Deliv. 2014, 11, 711–721.
- Song, K.-H.; Harvey, B.K.; Borden, M.A. State-of-the-Art of Microbubble-Assisted Blood-Brain Barrier Disruption. Theranostics 2018, 8, 4393–4408.
- Bader, K.B.; Holland, C.K. Gauging the Likelihood of Stable Cavitation from Ultrasound Contrast Agents. Phys. Med. Biol. 2013, 58, 127–144.
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193.
- Marty, B.; Larrat, B.; Van Landeghem, M.; Robic, C.; Robert, P.; Port, M.; Le Bihan, D.; Pernot, M.; Tanter, M.; Lethimonnier, F.; et al. Dynamic Study of Blood-Brain Barrier Closure after Its Disruption Using Ultrasound: A Quantitative Analysis. J. Cereb. Blood Flow Metab. 2012, 32, 1948–1958.
- Arsiwala, T.A.; Sprowls, S.A.; Blethen, K.E.; Fladeland, R.A.; Wolford, C.P.; Kielkowski, B.N.; Glass, M.J.; Wang, P.; Wilson, O.; Carpenter, J.S.; et al. Characterization of Passive Permeability after Low Intensity Focused Ultrasound Mediated Blood–Brain Barrier Disruption in a Preclinical Model. Fluids Barriers CNS 2022, 19, 72.
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69.
- Kovacs, Z.I.; Kim, S.; Jikaria, N.; Qureshi, F.; Milo, B.; Lewis, B.K.; Bresler, M.; Burks, S.R.; Frank, J.A. Disrupting the Blood-Brain Barrier by Focused Ultrasound Induces Sterile Inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E75–E84.
- Daneman, R.; Prat, A. The Blood-Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412.
- Chen, K.-T.; Wei, K.-C.; Liu, H.-L. Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Front. Pharm. 2019, 10, 86.
- Roberts, J.W.; Powlovich, L.; Sheybani, N.; LeBlang, S. Focused Ultrasound for the Treatment of Glioblastoma. J Neurooncol 2022, 157, 237–247.
- Kyriakou, A.; Neufeld, E.; Werner, B.; Székely, G.; Kuster, N. Full-Wave Acoustic and Thermal Modeling of Transcranial Ultrasound Propagation and Investigation of Skull-Induced Aberration Correction Techniques: A Feasibility Study. J. Ther. Ultrasound. 2015, 3, 11.
- Chen, K.-T.; Lin, Y.-J.; Chai, W.-Y.; Lin, C.-J.; Chen, P.-Y.; Huang, C.-Y.; Kuo, J.S.; Liu, H.-L.; Wei, K.-C. Neuronavigation-Guided Focused Ultrasound (NaviFUS) for Transcranial Blood-Brain Barrier Opening in Recurrent Glioblastoma Patients: Clinical Trial Protocol. Ann. Transl. Med. 2020, 8, 673.
- Epelbaum, S.; Burgos, N.; Canney, M.; Matthews, D.; Houot, M.; Santin, M.D.; Desseaux, C.; Bouchoux, G.; Stroer, S.; Martin, C.; et al. Pilot Study of Repeated Blood-Brain Barrier Disruption in Patients with Mild Alzheimer’s Disease with an Implantable Ultrasound Device. Alzheimers Res. Ther. 2022, 14, 40.
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801.
- Assistance Publique—Hôpitaux de Paris. A Study to Evaluate the Safety of Transient Opening of the Blood-Brain Barrier by Low Intensity Pulsed Ultrasound with the SonoCloud Implantable Device in Patients with Recurrent Glioblastoma before Chemotherapy Administration. 2018. Available online: Clinicaltrials.gov (accessed on 18 March 2023).
- Brandes, A.A.; Basso, U.; Vastola, F.; Tosoni, A.; Pasetto, L.M.; Jirillo, A.; Lonardi, S.; Paris, M.K.; Koussis, H.; Monfardini, S.; et al. Carboplatin and Teniposide as Third-Line Chemotherapy in Patients with Recurrent Oligodendroglioma or Oligoastrocytoma: A Phase II Study. Ann. Oncol. 2003, 14, 1727–1731.
- Yung, W.K.; Mechtler, L.; Gleason, M.J. Intravenous Carboplatin for Recurrent Malignant Glioma: A Phase II Study. J. Clin. Oncol. 1991, 9, 860–864.
- Poisson, M.; Péréon, Y.; Chiras, J.; Delattre, J.Y. Treatment of Recurrent Malignant Supratentorial Gliomas with Carboplatin (CBDCA). J. Neurooncol. 1991, 10, 139–144.
- Prados, M.D.; Warnick, R.E.; Mack, E.E.; Chandler, K.L.; Rabbitt, J.; Page, M.; Malec, M. Intravenous Carboplatin for Recurrent Gliomas. A Dose-Escalating Phase II Trial. Am. J. Clin. Oncol. 1996, 19, 609–612.
- Lunardi, P.; Osman Farah, J.; Mastronardi, L.; Puzzilli, F.; Lo Bianco, F.M. Intravenous Administration of High Doses of Carboplatin in Multimodal Treatment of High Grade Gliomas: A Phase II Study. Acta Neurochir. 1996, 138, 215–220.
- Murray, L.J.; Bridgewater, C.H.; Levy, D. Carboplatin Chemotherapy in Patients with Recurrent High-Grade Glioma. Clin. Oncol. (R Coll. Radiol.) 2011, 23, 55–61.
- Roci, E.; Cakani, B.; Brace, G.; Bushati, T.; Rroji, A.; Petrela, M.; Kaloshi, G. Platinum-Based Chemotherapy in Recurrent High-Grade Glioma Patients: Retrospective Study. Med. Arch. 2014, 68, 140–143.
- White, E.; Bienemann, A.; Pugh, J.; Castrique, E.; Wyatt, M.; Taylor, H.; Cox, A.; McLeod, C.; Gill, S. An Evaluation of the Safety and Feasibility of Convection-Enhanced Delivery of Carboplatin into the White Matter as a Potential Treatment for High-Grade Glioma. J. Neurooncol. 2012, 108, 77–88.
- Sonabend, A.M.; Gould, A.; Amidei, C.; Ward, R.; Schmidt, K.A.; Zhang, D.Y.; Gomez, C.; Bebawy, J.F.; Liu, B.P.; Bouchoux, G.; et al. Repeated Blood-Brain Barrier Opening with an Implantable Ultrasound Device for Delivery of Albumin-Bound Paclitaxel in Patients with Recurrent Glioblastoma: A Phase 1 Trial. Lancet. Oncol. 2023, 24, 509–522.
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant Anti-PD-1 Immunotherapy Promotes a Survival Benefit with Intratumoral and Systemic Immune Responses in Recurrent Glioblastoma. Nat. Med. 2019, 25, 477–486.
- InSightec. A Randomized Pivotal Study Assessing the Efficacy of Targeted Blood-Brain Barrier (BBB) Disruption Using Exablate Focused Ultrasound during the Standard of Care Treatment of Brain Metastases of Non-Small Cell Lung Cancer (NSCLC) Origin. 2022. Available online: Clinicaltrials.gov (accessed on 6 December 2022).
- Lozano, A.M. Safety and Feasibility of Focused Ultrasound-Enabled Liquid Biopsy in Patients with Brain Tumours. 2021. Available online: Clinicaltrials.gov (accessed on 9 April 2023).
- NaviFUS Corporation. An Open Label, Prospective, Pilot Study to Evaluate the Efficacy and Safety of Best Physician’s Choice of Standard of Care Combined with NaviFUS System in Patients with Recurrent Glioblastoma Multiforme. 2021. Available online: Clinicaltrials.gov (accessed on 24 May 2023).
- Assistance Publique—Hôpitaux de Paris. Multisite Open-Label Randomized Phase II Clinical Trial in Newly Diagnosed Glioblastoma Treated by Concurrent Temoradiation and Adjuvant Temozolomide +/- Ultrasound-Induced Blood Brain Barrier Opening. 2022. Available online: Clinicaltrials.gov (accessed on 28 March 2023).
- Lee, Y.; Choi, Y.; Park, E.-J.; Kwon, S.; Kim, H.; Lee, J.Y.; Lee, D.S. Improvement of Glymphatic–Lymphatic Drainage of Beta-Amyloid by Focused Ultrasound in Alzheimer’s Disease Model. Sci. Rep. 2020, 10, 16144.
More
