Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Lucas Fornari Laurindo.
The dragon fruit of pitaya is a rustic fruit belonging to the Cactaceae family, the genus Hylocereus. It is known as dragon fruit due to the presence of bright red skin with overlapping green fins covering the fruit. Other common names given to this fruit are pitahaya, dragon pearl fruit, night-blooming cereus, strawberry pear, and Cinderella plant. Depending on the species, its fruits may have different characteristics, such as shape, presence of thorns, skin, and pulp color, reflecting high genetic variability. The health-promoting potential of pitaya fruit is due to the presence of bioactive compounds related to numerous benefits such as anti-diabetic, anti-inflammatory, antioxidant, anti-cancer, and antimicrobial. As a result of these beneficial actions, the consumption of this fruit has increased in different regions worldwide.
- dragon fruit
- pitaya
- antioxidant
- anti-inflammatory
- health effects
- delivery system
1. Introduction
Dragon fruit has been used as a medicinal food since ancient times by Mayas—using the fruit and the flowers as a hypoglycemic, wound disinfectant and diuretic, for dysentery, tumor dissolution, and as a healing agent. Moreover, the flowers and the seeds can be used as beverages for gastritis, as laxatives, and to improve kidney function. These properties are derived from the multi-bioactive compounds found in this plant, mainly in the fruit. Furthermore, it shows no toxicity since a study with dragon fruit extract at a dose of 1250 to 5000 mg/kg did not produce interferences or abnormalities in the organs of animal models [65,66,67,68][1][2][3][4]. Below it is possible to find some effects of pitaya fruit.
As the results found in Table 1 show, the use of pitaya pulp or its bioactive compounds can exert anxiolytic, antioxidant, and anti-inflammatory effects in animal models. Improvements in hepatic steatosis and reduction of liver injury are also observed, in addition to balancing the intestinal microbiota. These results in animal studies allow us to say they can also be observed in humans.
Table 1.
In vivo biological and pharmacological activities of various pitaya extracts and pure compounds.
| Properties | Plant Part or Active Compounds | Species | Extraction | Experimental Model | Dose | Route of Administration | Methods of Analysis | Observations | References |
|---|---|---|---|---|---|---|---|---|---|
| Anxiolytic-like effects |
Hylocereus species. ↑—increase; ↓—decrease; HDL-c—high-density lipoprotein cholesterol; LDL-c—low-density lipoprotein cholesterol.
Table 3.
In vitro biological and pharmacological activities of various pitaya extracts and pure compounds.
| Properties | Plant Part Used or Compounds | Species | Models | Type of Extract | Tested Concentrations | Methods | Observations | References |
|---|---|---|---|---|---|---|---|---|
| Pulp and peel rich in maltotriose, quercetin-3-O-hexoside, and betalains bioactive compounds. | H. polyrhizus | . | Ethanolic and aqueous extracts. | |||||
| Anti-glycation | Freeze-dried powder rich in phenolic bioactive compounds. | Zebrafish from both sexes. | 0.1 mg/mL or 0.5 mg/mL or 1.0 mg/mL, 20 μL. | Dissolved in the fish’s water. | Anxiolytic activities and toxicity assays. | |||
| 19 | H. polyrhizus | . | Bovine serum albumin. | Ethanolic, hydro-ethanolic, methanolic, hydro-methanolic, acetone, aqueous, and petroleum ether extracts. | Concentrations range from 20 µg/mL to 100 µg/mL. | Glycation and aggregation of protein assays, carbohydrate cleaving enzyme inhibitory activity assessments, antioxidant studies, and determination of fructosamine inhibition. | Methanolic and acetone extracts were the most significant anti-glycation and antioxidant agents. The potential polyphenolic compounds associated with these effects were mainly 4-prenylresveratrol, vicenin, and luteolin. | [88][67] |
| Leaves rich in triterpenes. | H. undatus. | Bovine serum albumin. | Chloroform extract. | 0.5–2.0 mg·mL−1, 3 mmol·L−1, and 5 mmol·L−1. | Protein glycation and aggregation assessments. | The studied triterpenes could inhibit protein glycation at multiple stages, decreasing protein oxidation and protecting against diabetic-related complications. | [89][68] |
Based on the inclusion and exclusion criteria for the search of studies that were performed using pitaya in humans, only five clinical trials were added to Table 4. The risk of bias observed in these studies is found in Table 5. These trials are commented on below.
Table 4.
Effects of the genus
Hylocereus
in human studies.
| Reference | Local | Model and Patients | Intervention | Outcomes | Adverse Effects | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prognostics or Demographic | Characteristics | Outcomes | Intention to Treat Analysis | Sample Calculation | Adequate Follow-Up | ||||||||||||||||||||
| Anti-diabetic | |||||||||||||||||||||||||
| Bulb and peel. | |||||||||||||||||||||||||
| [20][76] | United Kingdom | Randomized, double-blind, placebo-controlled, cross-over trial with 19 healthy subjects (8 ♂, 11 ♀; 18–40 y) | Participants consumed 24 g of whole dragon fruit powder (33 mg of betalains) or a placebo daily/for 14 days. Blood pressure, Flow-mediated dilation (FMD), and arterial stiffness were evaluated (0, 1, 2, 3, and 4 h and 14 days after daily consumption). | Pitaya consumption significantly improved acute FMD at 2, 3, and 4 h post-consumption compared with placebo. This was also observed until 14 d. Pulse wave velocity was acutely significantly decreased at 3 h, and the augmentation index improved after 14 days compared with the placebo. No significant differences were observed for BP. | H. undatus. | ||||||||||||||||||||
| [20][76 | Not reported by patients. | The extracts showed no toxicity in the fish model and exerted significant anxiolytic effects as the fish reduced their permanence in the clear zone of the experimentation area compared to controls. | ] | Anthocyanins (cyanidin 3-glucoside, delphinidin 3-glucoside, and pelargonidin 3-glucoside) | [ | 21 | Peel and pulp | ] | [5 | ||||||||||||||||
| ] | ] | ||||||||||||||||||||||||
 |
Anti-inflammatory, antioxidant | 0.82 ± 0.17 a 12.67 ± 0.63 mg/100 g (pulp) and 44.3865 ± 1.3125 mg/100 g (peel) | Yes | Metabolic effects | |||||||||||||||||||||
| Yes | Yes | Yes | Yes | Yes | Yes | NR | NR | Yes | [6,Betalain-rich pitaya pulp. | 47H. undatus. | Aqueous extract. | ,Streptozotocin-induced diabetes in male Sprague-Dawley rats. | 250 or 500 mg per kg body weight. | 48,49][20][ | [19][64]21][22] | Indonesia | |||||||||
| [19 | [23] | ] | Ascorbic acid | Intragastric gavage. | Pulse wave velocities, surgical procedures, and oxidative stress analyses. | The treated rats had reduced blood pressure, pulse wave velocities, and pulse pressures. | Quantitative research type quasi-experiment (pre-test and post-test nonequivalent control group) with 32 students (4 ♂, 28 ♀; 21–26 y) who presented excess nutritional status. | [ | 22][6] | ||||||||||||||||
| Peel and pulp |  |
Antioxidant, anti-cancer, anti-diabetic, anti-lipidemic | 2.94 ± 1.1 a 5.64 ± 0.7 mg/100 g (pulp) and 175 ± 15.7 µmol Trolox equivalents antioxidant capacity (TEAC)/g (peel) | [ | Participants consumed 180 g of red pitaya/7 days. | The consumption of dragon fruit effectively reduced glycemia and blood pressure in subjects with excess nutritional status. | Not reported by the authors. | ||||||||||||||||||
| 64] | Yes | No | No | No | NR | Yes | Yes | NR | NR | No | Betalain-rich pitaya juice. | H. polyrhizus. | Aqueous juice. | High-carbohydrate, high-fat diet-induced metabolic syndrome male Wistar rats. | 5% of red pitaya juice during the diet. | ||||||||||
| [ | Oral by feeding. | 49 | Biochemical and physical tests, as well as histopathological assessments. | There was a significant reduction in the diastolic stiffness of the treated rats. Additionally, the treated rats presented reductions in the alkaline phosphatase and alanine transaminase serum concentrations. | ,50][23Dipeptidyl peptidase-IV enzyme. | [ | 26 | ] | [7] | ||||||||||||||||
| ][24 | [ | Ethanolic and hydro- ethanolic extracts of pitaya’s fruit and ethanolic extract of pitaya’s peel. | 17] | Betacyanin | Peel and pulp |  |
Anti-microbial, anti-viral, pigments, hypolipidemic | ][10 mg/100 µL. | 61]10.3 ± 0.22 a 82.79 ± 0.55 (pulp) and 13.8 ± 0.85 a 18.67 ± 0.50 mg/100 g (peel) | In vitro dipeptidyl peptidase-IV enzyme inhibition. |
The hydro-ethanolic extract of pitaya’s fruit exerted 26.8 ± 0.55% of DPP-IV inhibition, the ethanolic extract of pitaya’s peel exerted 62.3 ± 0.63%, and the ethanolic extract of pitaya’s fruit exerted 84.2 ± 0.72%. | ||||||||||||||
| [17 | Malaysia | ][61 | Randomized trial with 28 subjects (14 ♂, 14 ♀, 21 with type 2 diabetes; 20–55 y) | [ | 90 | ] | [ | 69] | |||||||||||||||||
| ] | Participants were divided into 4 groups: 400 g of red pitaya/d, 600 g of red pitaya/d, negative control (diabetic subjects with normal diet), and the last group (healthy subjects with normal diet)/7 w. The study included 4 weeks of treatment and 2 weeks of wash-out. | After 4w: Group 1 showed a significant increase in HDL-c and a significant reduction of glycemia, LDL-c, and triglyceride. There was a significant augment of total cholesterol level on 7th w. No significant modifications were seen in group 2. No significant modifications were seen in weight and body fat in any group. | Not reported | ||||||||||||||||||||||
| Yes | No | NR | NR | NR | Pitaya’s juice. | H. polyrhizus. | Aqueous juice. | High-carbohydrate, high-fat diet-induced metabolic syndrome male Wistar rats. | 5% of red pitaya juice during the diet. | Oral by feeding. | |||||||||||||||
| Yes | Yes | [51 | Biochemical and physical tests, as well as genetic assessments. | ,52] | During the treatment, the omental and epididymal fat of the rats increased. The pitaya treatment reversed the rats’ metabolic changes by up-regulating the obesity-related Pomc and Insr genes in the liver tissues. | [25][26] | Lycopene | [ | 27][8] | ||||||||||||||||
| No | No | Yes | Pulp | Anti-plasmodium |  |
Peel. | H. polyrhizus. | Plasmodium falciparum 3D7 and W2 strains, HeLa cells, and SK-OV-3 cells. | Pigmented, n-hexane, dichloromethane, and ethyl acetate extracts. | - | Anti-plasmodium and cytotoxicity assays. | The dichloromethane extract demonstrated the most prominent anti-plasmodium activity at concentrations of 2.13 ± 0.42 µg/mL. The extracts showed cytotoxicity against the cancer cells at final concentrations of more than 1000 µg/mL. | [16][91][70] | ||||||||||||
| [43] | Malaysia | Single-blinded cross-over trial with 36 Pre-diabetic participants | Pre-diabetic participants received 60, 80 or 100 g of spray pitaya (red) powder daily/4 week. | ||||||||||||||||||||||
| [16] | A significant decrease was seen in glycemia, total cholesterol, LDL-c, and triglycerides. A significant increase in HDL-c and total antioxidant status was observed. | [43] | Antioxidant, anti-diabetic, anti-cancer | Not reported | |||||||||||||||||||||
| Yes | 3.2 ± 0.6 µg/100 g (pulp) | No | Yes | No | NR | No | Yes | No | No | Yes | Pitaya’s peel purified betacyanins. | H. undatus. | Cytotoxicity | Pulp and peel rich in maltotriose, quercetin-3-O-hexoside, and betalains bioactive compounds. | H. polyrhizus. | Vero cell lines. | Ethanolic and aqueous extracts. | - | [Cytotoxicity assays. | 18][The toxicity against the cells ranged from 2.18 to 2.36 mg/mL for the peel and pulp. | 44][21] | Malaysia[ | Single-blinded cross-over trial with 60 normocholesterolemic subjects5 | Participants were divided to receive 3, 4 or 5 sachets of spray pitaya (red) powder (20 g each) daily/4 weeks.] | |
| Significant reduction in glycemia, total cholesterol, LDL-c, and triglycerides. A significant increase in HDL-c and total antioxidant status. | Not reported by the participants. |
HDL-c: High-density lipoprotein; LDL-c: Low-density lipoprotein.
Table 5.
Description of the biases observed in the randomized clinical trials performed with the genus
Hylocereus
.
| Study | Question Focus | Appropriate Randomization | Allocation Blinding | Double-Blind | Losses (<20%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [ | ||||||||||||||||
| 18 | ||||||||||||||||
| ] | ||||||||||||||||
| [44] | Yes | No | Purified betacyanins. | High-fat diet-fed male C57BL/6J mice. | Different concentrations of different purified betacyanins. | Oral by feeding. | ||||||||||
| [6,52,53 | Biochemical and histopathological analyses. | ][ | The purified betacyanin ameliorated the AT’s hypertrophy, liver steatosis, body glucose intolerance, and the body’s IR. In the liver, the purified betacyanins also augmented the genetic expression of lipid metabolism genes, such as the Acox1, Cpt1b, Cpt1a, Insig1, | PPARγ, AdipoR2, and Insig2 and of FGF-21 genes. The purified betacyanins alleviated the liver’s FGF21 resistance, decreased the liver’s | fatty acid biosynthesis, and elevated the liver’s fatty acid oxidation. | 20][26][27] | [28][ | β-carotene9] | ||||||||
| Pulp | 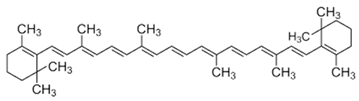 |
Antioxidant, anti-diabetic, anti-cardiovascular diseases | 209.1 ± 0.1 µg/100 g (pulp) | Yes | No | NR | No | Yes | No | No | Yes | Betacyanin-rich pitaya fruit. | H. polyrhizus. | Red pitaya’s fruit betacyanins. | Diet-induced obesity, liver steatosis, and insulin resistance C57BL/6J mice. |
|
| [10,54][28][29] | Tocopherol | Seed | 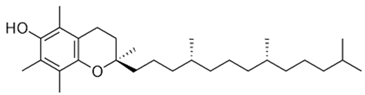 | 200 mg/kg. | Intragastric gavage. | Biochemical, sequencing, and histological analyses. | The fruit protected the mice from obesity and its related metabolic disorders. There were improvements in the inflammatory statuses of the treated rats, as well as their gut microbiota (there was a decrease in the ratio of | Firmicutes and Bacteroidetes and an increase in the relative amount of Akkermansia). | [31][10] | |||||||
| Antioxidant, anti-cancer, anti-diabetes, anti-cardiovascular diseases | 150 µg/100 g (seed) | Peel. | H. polyrhizus and H. undatus. | PC3, Bcap-37, and MGC-803 cell lines. | Supercritical carbon dioxide extracts. | Maximum concentrations of 0.7 mg/mL. | Cytotoxicity assays. | All extracts demonstrated significant cytotoxicity against the cancer cell lines at concentrations ranging from 0.61 to 0.73 mg/mL. | [36][71] | Pitaya juice rich in polyphenols and flavonoids bioactive compounds. | H. undatus. | Aqueous juice. | Steatosis diet-induced obese C57BL/6J mice. | - | Oral by drinking. | |
| [6,55][ | Biochemical and histopathological assessments. | 20 | There were improvements in the FGF-21 resistance and lipid metabolisms of the treated rats. Additionally, the juice protected the rats against hepatic steatosis and IR. | ][30] | [ | p-Coumaric acid | 32 | Pulp and seed | 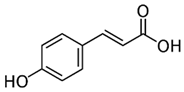 | ] | Antioxidant, anti-diabetic, anti-lipidemic[ | |||||
| Hepatoprotective | 11 | ] | ||||||||||||||
| 0.365 a 1.52 ± 1,4 mg/100 g (seed) and 0.16 mg 100 g (pulp) | Peel rich in betacyanins. | H. undatus. | HepG2 cells. | Pressurized in the hot water extract. | Concentrations range from 20 μg/mL to 100 μg/mL. | Measurements of ROS and lipid accumulation, as well as biochemical tests. | The betacyanin-rich extracts could effectively exert antioxidant and hepatoprotective effects in the treated cells by controlling their lipid metabolisms and diminishing their TG contents. These compounds inhibited the liberation of the liver enzymes AST and ALT and probably regulated the mRNA expressions of the fatty acid synthase and carnitine palmitoyl transferase 1. | [92][72 | ||||||||
| ] | [10Concentrated Pitaya Pulp with seeds | ,49,H. polyrhizus. | ||||||||||||||
| Anti-viral | Pulp with seed | Hyperlipidemic female C57BL/6 mice | 56 | 100, 200, and 400 mg/kg/day | Oral by feeding | , | Biochemical assessments. | There was an increase in the levels of HDL-c and a significant reduction in the levels of Total cholesterol, LDL, triglycerides, glycemia, AST, and ALT. | 57][23][28][31][32][23][12] | |||||||
| Gallic acid | Peel, pulp, and seed | 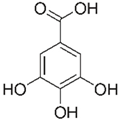 |
Antioxidant, anti-diabetic, anti-obesity | 24.0 ± 2.7 a 53.2 ± 6.2 mg GAE/100 g (pulp), 39.9 mg GAE/100 g (peel)and 375.1 mg GAE/100 g (seed) | Betacyanin-rich pitaya peel extract. | H. polyrhizus. | Methanolic extract. | Alcoholic-progressive liver disease ethanolic-diet C57BL/6 mice. | ||||||||
| [ | 500 and 1000 mg/kg of body weight. | 6,58 | Intragastric gavage. | ][20 | Biochemical and histopathological assessments. | ][33 | The treated group presented diminished liver injury and improved liver lipid metabolism via decreases in the SREBP-1 and increases in AMPK and PPAR-α protein expressions. The extract also inhibited the Nrf2 and CYP2E1 expression, reduced endotoxin levels, and decreased TLR4, MyD88, TNF-α, and IL-1β expression in the treated rats’ liver. | ] | [25][13] | Syringic acid | Peel, pulp, and seed | 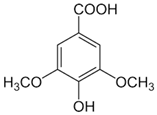 |
Antioxidant, anti-diabetes, anti-cardiovascular diseases, anti-cancer | Variando de 0.095 a 65.10 ± 0.04 µg/100 g (peel and pulp) Seed (NA) |
||
| [5 | Concentrated Pitaya Pulp with seeds | H. polyrhizus. | Pulp with seed | ,Hyperglycemic Zebrafish | 5–100% of pitaya pulp | 49Oral by feeding | Biochemical assessments. | ,There was a significant decrease in glycemia in all concentrations compared to placebo and with the use of metformin. | 59][23][34][35][24][14] | |||||||
| Vanillic acid | Peel and pulp | 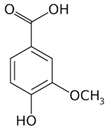 |
Antioxidant, anti-inflammatory, anti-diabetic, anti-proliferative, and anti-atherogenic activities | 0.64 mg/100 g (peel and pulp) | Gastrointestinal | Pitaya’s fruit extract. | H. polyrhizus. | Ethanolic extract. | Balb/c mice induced colitis by trinitrobenzene sulphonic. | 1 g/kg. | Intraperitoneally. | Biochemical and histopathological analysis. | The extract exerted anti-inflammatory (decreases in the Ikb-a degradation and nuclear NF-kb protein levels) effects and prevented colitis development (reduced histological damage score) in the treated mice. | [29][15] | ||
| Wound-healing | Pitaya’s leaves, rind, fruit pulp, and flower extracts. | H. undatus. | Aqueous extract. | Streptozotocin diabetic Wistar rats | 200 µL/wound at concentrations of 0.05%, 0.1%, 0.2%, 0.4% and 0.5% twice daily. |
Topically. | Wound healing assays and DNA and protein estimation. | The use of the extract facilitated wound healing by enhancing tensile strength, hydroxyproline, DNA, total proteins, collagen content, and epithelization. | [30][16] |
ALT—Alanine transaminase; AMP—adenosine monophosphate; AMPK—AMP-activated protein kinase; AST—aspartate aminotransferase; AT—adipose tissue; CYP2E1—cytochrome P450 2E1; FGF-21—fibroblast growth factor 21; Ikb-a—Ikb kinase alpha; Insr—insulin receptor gene; IL-1β—interleukin 1 beta; IR—insulin resistance; MyD88—myeloid differentiation primary response gene 88; NF-kb—nuclear factor kappa b; Nrf2—nuclear factor erythroid 2-related factor 2; PPAR-α—peroxisome proliferator-activated receptor; Pomc—proopiomelanocortin gene; SREBP-1—hepatic sterol regulatory element-binding protein 1c; TLR4—toll-like receptor 4; TNF-α—tumor factor necrosis alfa.
2. Antioxidant Effects by Species
Hylocereus. polyrhizus is rich in betalains and other bioactive compounds such as vitamins and phenolic compounds that exert relevant antioxidant properties and, for these reasons, are related to the prevention of several human diseases. The oil results from the seeds, and the peel is also an essential source of antioxidant compounds. The peel of H. undatus possesses more flavonoids than the flesh [65,66,67,69][1][2][3][17] (Table 2).
Table 2.
Main bioactive compounds of pulp, peel, and seeds of the genus
Hylocereus
.
| References | Bioactive Compounds | Pulp, Peel, or Seed | Molecular Structures | Health Effects | Concentration (Dry Weight) |
|---|---|---|---|---|---|
| [45,46][18][ | |||||
| [ | |||||
| 6 | |||||
| ] | |||||
| [ | |||||
| 20 | |||||
| ] | |||||
| Phthalic acid | |||||
| Peel | 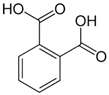 |
Antioxidant | (NA) | ||
| [39][36] | Benzoic acids derivatives: salicylic acid (a), 3- hydroxyl benzoic acid (b), 4-hydroxy benzoic acid (c). |
Peel and Pulp | 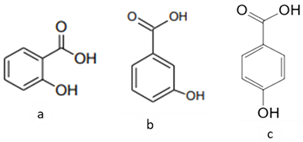 |
Antioxidant | Variando de 0.48 ± 0.0 a 11.97 ± 0.8 µg/100 g (pulp and peel) |
| [6,49,60][20][23][37] | Caffeic acid | Peel and seed | 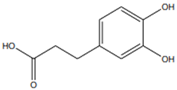 |
Antioxidant | 0.08 mg/100 g (pulp and peel) |
| [45,61][18][38] | Quercetin | Peel and pulp | 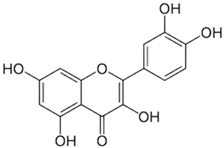 |
Antioxidant, anti-inflammatory, anti-diabetic | 3.43 mg/100 g (pulp) Peel (NA) |
| [44,48,62,63,64][22][39][40][41][42] | Fatty acids: oleic acid (a), linoleic acid (b), linolenic acid (c), and palmitic acid (d) |
Pulp and seeds | 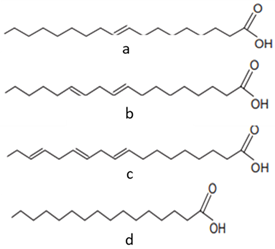 |
Nutraceutical activity | 129.11 (a), 252.65 (b), 5.33 (c), 62.74 (d) mg/100 g (pulp) and 60 (a), 21 (c), 13 (d) g/100 g (seeds) |
The antioxidant properties of dragon fruit extract were investigated. Total antioxidant status was reduced in pre-diabetic and normocholesterolemic subjects that consumed red pitaya [16,18][43][44]. Harahap and Amelia [70][45] showed that fruit extract could minimize oxidative damage production in animals submitted to physical exercises. Other authors have shown that the pulp extract can decrease oxidative damage in STZ-induced diabetes in rats [71][46].
Putri et al. [72][47] showed that the intake of red dragon fruit could reduce malondialdehyde levels in diabetic rats and, for these reasons, is associated with reducing the oxidative stress related to this disease. Figure 1 shows the main antioxidant effects associated with the Hylocereus species.

Figure 1.
Main antioxidant effects of
Hylocereus
species and their health effects. ↑—increase; ↓— decrease.
3. Anti-Inflammatory Effects
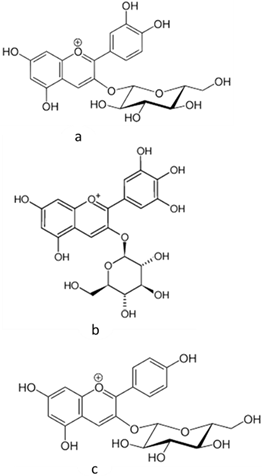
Besides the antioxidant actions, dragon fruit can also exert anti-inflammatory actions. Saenjum et al. [45][18] found anthocyanins (cyanidin 3-glucoside, delphinidin 3-glucoside, and pelargonidin 3-glucoside) in the pulp and peel of pitaya red. The authors found that the pulp enriched with the first anthocyanin (cyanidin 3-glucoside) inhibited the synthesis of reactive oxygen and nitrogen species, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS), in in vitro models and without resulting in cytotoxicity.
Another study showed that the dragon flesh and peel extract and the isolated squalene led to the inhibition of pro-inflammatory enzymes such as cyclooxygenase-2 lipoxygenase and acetylcholinesterase and concluded that this fruit could produce a significant potential for the control and management of inflammatory processes through different pathways that may include, prostaglandin, leukotriene, and cholinergic pathways [73][48].
Amim et al. [74][49] found different effects (anti-inflammatory, antioxidant and anti-bacterial actions) in the extracts of H. polyrhizus and H. undatus (the aqueous extract of H. polyrhizus showed most significant effects than H. undatus). The authors found that the aqueous extract of these species resulted in more protective anti-inflammatory and antioxidant activities compared to the ethyl acetate and ethanolic extracts.
Al-Radadi et al. [75][50] found significant anti-inflammatory, anti-diabetic, anti-Alzheimer, and cytotoxic effects of gold nanoparticles using H. polyrhizus.
Figure 2 shows the most prominent anti-inflammatory effects associated with the Hylocereus species.
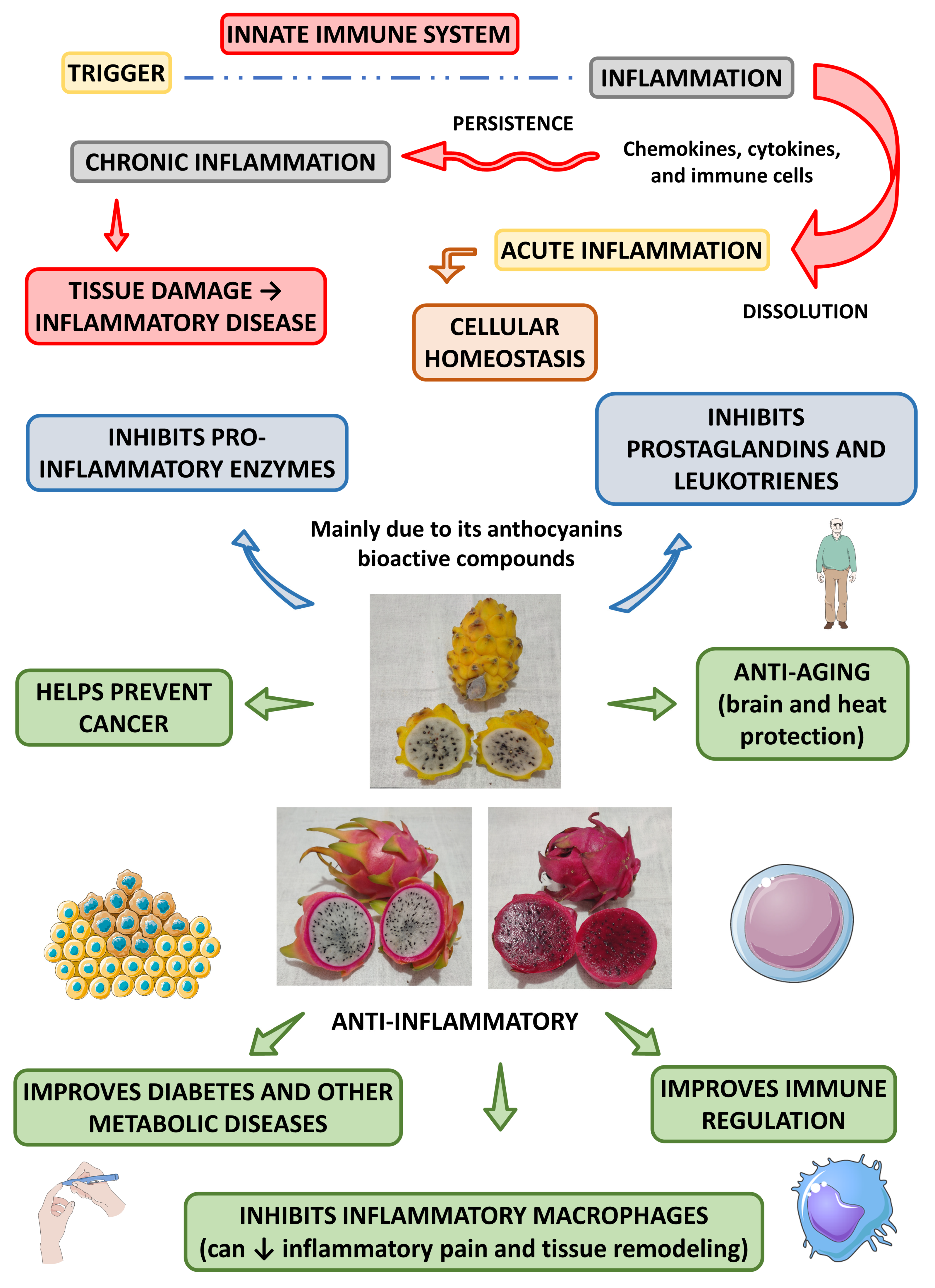
Figure 2.
Main anti-inflammatory effects of
Hylocereus
species and their health effects. ↓—decrease.
4. Prebiotic Effects
The main carbohydrates in white and red flesh dragon fruit are glucose, fructose, and oligosaccharides. The mixed oligosaccharides are resistant to hydrolysis by human α-amylase and artificial human gastric juice (about 34.88% and 4.04%, respectively). Moreover, the mixed oligosaccharides can also stimulate the growth of lactobacilli and bifidobacterial, thus showing prebiotic properties [76][51].
Dasaesamoh et al. [77][52] showed that the fecal fermentation of pitaya oligosaccharides increased the populations of Bifidobacteria and Lactobacillus and reduced the populations of Bacteroides and Clostridium. Moreover, this fecal fermentation resulted in a positive prebiotic effect. Lactic acid, acetic acid, propionic, and butyric acids were produced at substantial concentrations.
5. Antimicrobial Effects
In a study to investigate the antimicrobial effect of red pitaya peels, Temak et al. [78][53] found that the extract has efficient in vivo and in vitro effects against several microorganisms, such as Escherichi coli and P. aeruginosa.
Sushmitha et al. [79][54] investigated the effects of H. undatus seeds in Gram-negative and Gram-positive bacterial species and found that the minimum inhibitory concentration is 50 µL for Staphylococcus aureus and Escherichia coli. Tenore et al. [80][55] also found antimicrobial activity for hexane, chloroform, and ethanol extract of the skin of H. undatus and showed inhibition of the growth of Gram-negative and Gram-positive bacteria.
Extract of the peel and fruit of the red dragon fruit can also exert anti-fungal actions against Rhizoctonia solani, Candida albicans, Aspergillus flavus, Botrytis cinerea, Fusarium oxysporum, and Cladosporium herbarum [80][55].
6. Anti-Cancer Effects
Some studies have shown the anti-cancer potential of dragon fruit. Divakaran et al. [81][56] aimed to evaluate the ability of this fruit to produce nanoparticles and found they can significantly inhibit the growth of MCF-7 breast cancer cells.
Another study showed that the fecal fermentation of pitaya oligosaccharides augmented the populations of Lactobacillus and decreased the populations of Bacteroides and Clostridium, and resulted in the production of lactic acid, acetic acid, propionic and butyric acids that can inhibit Caco-2 cells and has a potential for risk reduction in colon cancer [77][52].
In a very interesting study, Padmavathy et al. [37][57] demonstrated that methanol extracts of H. undatus have promising anti-cancer effects against human liver cancer (HepG-2) cells.
Wu et al. [82][58] investigated the anti-proliferative effect of red pitaya on B16F10 melanoma cells. They showed that the peel has stronger inhibition of the growth of these cancer cells than the flesh. The authors concluded that both peel and flesh are rich in polyphenols and good sources of antioxidants, and for these reasons, the peel can inhibit the growth of melanoma cells.
The anti-cancer activities promoted by pitaya are related to several bioactive compounds such as phenolic acids, flavonoids, and betacyanin [83,84][59][60].
Figure 3
shows the most pronounced anti-cancer effects associated with the
Hylocereus
species.
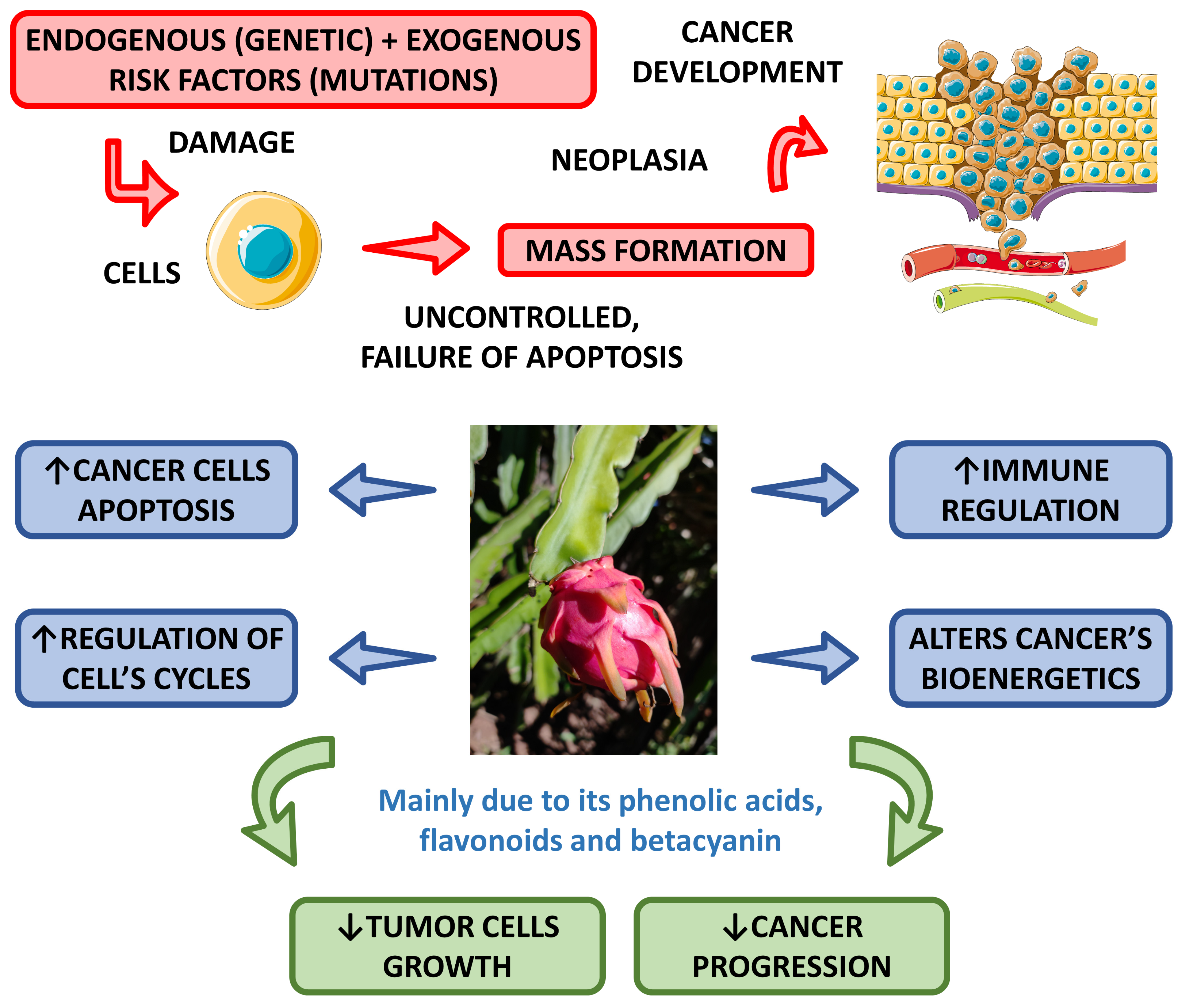
Figure 3.
Main anti-cancer effects of
Hylocereus
species. ↑—increase; ↓—decrease.
7. Anti-Diabetic Effects
Many studies have demonstrated that the consumption of red pitaya can reduce glycemia in humans [16,17,18][43][44][61]. In a systematic revisewarch and meta-analysis, Poolsup et al. [8][62] found that dragon fruit can be used to prevent diabetes.
The study of Putri et al. [72][47] showed that pitaya associated with metformin could significantly decrease glycemia and HOMA-IR (homeostasis model assessment-Insulin Resistance) in type 2 diabetic rats. The authors suggested that red dragon fruit could be used as an alternative to metformin due to its effectiveness in decreasing HOMA-IR (and thus, insulin resistance) and malondialdehyde levels. Moreover, the consumption of red pitaya promoted a hypoglycemic effect in dyslipidemic C57BL/6 mice, contributing to reducing the risk of insulin resistance [85][63].
Fadlilah and Sucipto [19][64] found that pitaya (H. polyrhizus) effectively reduces fasting blood sugar levels in students who consume high calories daily.
Marietta et al. [86][65] investigated the effects of red pitaya skin extract on glycemia and lipid profile of diabetic and dyslipidemic male Wistar rats and found no significant reduction in glycemia.
8. Anti-Lipidemic Effects
The use of red pitaya can improve lipid profile, decrease total cholesterol, LDL-c, and triglycerides, and increase HDL-c levels in normocholesterolemic subjects, pre-diabetic, and type 2 diabetic patients [16,17,18][43][44][61].
The consumption of red pitaya also showed benefits in lipid levels in dyslipidemic C57BL/6 mice, contributing to reducing cardiovascular diseases [85][63].
The study of Marietta et al. [86][65] evaluated the effects of the consumption of red pitaya skin extract on the lipid profile of male Wistar rats with diabetes and dyslipidemia and did not find a significant reduction in the lipid profile of these animals.
Setiawan et al. [87][66] investigated the effects of H. polyrhizus peel powder on male Balb-c mice. Their results showed decreased triglycerides, total cholesterol levels, and LDL-c. Moreover, they observed an increase in HDL-c levels. The authors concluded that the use of the peel of this fruit could improve the blood lipid of mice with hyperlipidemia.
Figure 4 shows the most relevant anti-lipidemic and anti-diabetic effects associated with the Hylocereus species and how these effects are associated with atherosclerosis. Table 3 summarizes some in vitro studies performed with pitaya.

Figure 4. Cardiovascular protective effects of the
| Pulp rich in betacyanins. | ||||||||
| H. polyrhizus | ||||||||
| . | ||||||||
| Denv-2 strains and Vero cells. | ||||||||
| Methanolic extracts. | ||||||||
| Maximum concentrations at 4.346 mg·mL | −1 | . | Cytotoxicity and anti-viral assays. | The extract was considered non-toxic for the cells at concentrations below 2.50 mg·mL | −1 | . The most prominent anti-viral potential (95.0% virus inhibition) against the denv-2 was achieved at concentrations of 126.70 μg mL | −1. | [93][73] |
| Immunomodulatory | Peel rich in the terpenoid lupeol. | H. polyrhizus. | Mice macrophages. | The compound was dry isolated. | 100, 50, 25, 12.5 and 6.25 µg/mL. |
Phagocytosis assessments and other macrophagic tests. | The terpenoid lupeol isolated from the mice was effectively associated with the increase of macrophage phagocytosis of latex beads, demonstrating potent immunomodulatory effects. | [94][74] |
| Bone effects | Fruit extract. | H. polyrhizus. | Bone marrow-derived mesenchymal stem cells. | Aqueous. | 50, 100, 200, 300, and 400 µg/mL. | Cells osteogenic and proliferation assessments. | The fruit extract could promote the osteogenic differentiation and proliferation of the treated cells. | [95][75] |
ALT—alanine aminotransferase; AST—aspartate aminotransferase; Bcap-37—human breast cancer cell line; Denv-2—dengue virus type 2; DPP-IV—dipeptidyl peptidase-IV; HeLa—cervical cancer cell line; HepG2—human hepatoma cell line; MGC-803—human gastric cancer cell line; mRNA—messenger ribonucleic acid; PC3—human prostate cancer cell line; ROS—reactive oxygen species; SK-OV-3—ovarian cancer cell line; TG—triglycerides.
9. The Effects of Dragon Fruit on Humans: Evaluation of Clinical Trials
NR—not reported.
Shafie et al. [18][44] investigated the effects of using spray pitaya powder (SPP) in normocholesterolemic subjects. All the doses led to reductions in total cholesterol and LDL-c (p < 0.05); however, the higher the dose, the higher the reduction in cholesterol levels. For the blood levels of triglycerides and HDL-c, there were significant differences (decrease and increase, respectively) in the higher dose (p < 0.05).
In the study of Akhiruddin [16][43], the pre-diabetic participants were allocated to receive pitaya powder (obtained from H. polyrhizus) in different concentrations. At the end of the treatment, compared to the control group, the group that received 100 g per day showed the best reduction in blood glucose (22.90%, p < 0.05). The same was observed for the lipid levels; there was a significant reduction (p < 0.05) in total cholesterol (26.44%), triglycerides (20.54%), and LDL-c (69.55%) (the higher the dose, the higher the percentage of reduction). Moreover, the group that received 100 g of the red pitaya powder presented an increase of 63.8% in HDL-c levels. Although the results of these two studies are very interesting, the authors did not mention the participants’ age and gender.
Abd Hadi et al. [17][61] investigated the effects of consuming pitaya fruit in type 2 diabetic subjects that receive red pitaya daily. Participants were subjected to a seven-week trial consisting of phase 1 (one week) and phase 2 (4 weeks) of treatment, and phase 3 (2 weeks of wash-out). The results showed a reduction (p < 0.05) in glycemia and lipids.
Fadlilah and Sucipto [19][64] investigated the effects of red pitaya intake on students’ blood pressure and glycemia after excessive food consumption. The obtention of the samples was performed using consecutive sampling techniques with thirty-two respondents each for the treatment and control groups. The glycemia pre-test and post-test in the treated group were 87.5 mg/dL and 81.0 mg/dL, respectively, and in the control group were 83.00 mg/d and 82.0 mg/dL, respectively. The systolic blood pressure pre- and post-test were 112 mmHg and 115 mmHg, respectively, in the control group, compared to 117 mmHg and 109 mmHg, in the intervention group. The diastolic blood pressure pre-test and post-test were 79 mmHg and 82 mmHg in the control group; in the treated group, it was 77 mmHg and 70 mmHg in the pre- and post-test, respectively.
The study performed by Cheok et al. [20][76] showed that pitaya intake significantly improved acute low-mediated dilation (p < 0.001) compared to placebo. Pulse wave velocity was significantly decreased (p = 0.003), whereas the augmentation index improved after fourteen days (p = 0.02). Although the results of this trial are interesting, it is biased due to the small sample and a short-time follow-up that could interfere with the interpretation of the results.
Since the pulp and the skin of Hylocereus can exert antioxidant and anti-inflammatory actions, the consumption of this plant, as shown by the trials mentioned above, can reduce the risk of several diseases related to oxidative stress and inflammatory processes. These diseases include diabetes, dyslipidemia, metabolic syndrome, and cardiovascular conditions.
References
- Hernández, Y.D.O.; Salazar, J.A.C. Pitahaya (Hylocereus spp.): A short review. Comun. Sci. 2012, 3, 220–237.
- Jeronimo, M.C.; Orsine, J.V.C.; Novaes, M.R.C.G. Nutritional pharmacological and toxicological characteristics of pitaya (Hylocereus undatus): A review of the literature. Afr. J. Pharm. Pharmacol. 2017, 11, 300–304.
- Safira, A.; Savitri, S.L.; Putri, A.R.B.; Hamonangan, J.M.; Safinda, B.; Solikhah, T.I.; Khairullah, A.R.; Puspitarani, G.A. Review on the pharmacological and health aspects of Hylocereus or Pitaya: An update. J. Drug Deliv. Ther. 2021, 11, 297–303.
- Kumar, S.B.; Issac, R.; Prabha, M.L. Functional and health-promoting bioactivities of dragon fruit. Drug Invent. Today 2018, 10, 3307–3310.
- Lira, S.M.; Dionísio, A.P.; Holanda, M.O.; Marques, C.G.; Silva, G.S.D.; Correa, L.C.; Santos, G.B.M.; de Abreu, F.A.P.; Magalhães, F.E.A.; Rebouças, E.L.; et al. Metabolic profile of pitaya (Hylocereus polyrhizus (F.A.C. Weber) Britton & Rose) by UPLC-QTOF-MS(E) and assessment of its toxicity and anxiolytic-like effect in adult zebrafish. Food Res. Int. 2020, 127, 108701.
- Anand Swarup, K.R.; Sattar, M.A.; Abdullah, N.A.; Abdulla, M.H.; Salman, I.M.; Rathore, H.A.; Johns, E.J. Effect of dragon fruit extract on oxidative stress and aortic stiffness in streptozotocin-induced diabetes in rats. Pharmacogn. Res. 2010, 2, 31–35.
- Ramli, N.S.; Brown, L.; Ismail, P.; Rahmat, A. Effects of red pitaya juice supplementation on cardiovascular and hepatic changes in high-carbohydrate, high-fat diet-induced metabolic syndrome rats. BMC Complement. Altern. Med. 2014, 14, 189.
- Ramli, N.S.; Ismail, P.; Rahmat, A. Red pitaya juice supplementation ameliorates energy balance homeostasis by modulating obesity-related genes in high-carbohydrate, high-fat diet-induced metabolic syndrome rats. BMC Complement. Altern. Med. 2016, 16, 243.
- Song, H.; Chu, Q.; Xu, D.; Xu, Y.; Zheng, X. Purified Betacyanins from Hylocereus undatus Peel Ameliorate Obesity and Insulin Resistance in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2016, 64, 236–244.
- Macias-Ceja, D.C.; Cosín-Roger, J.; Ortiz-Masiá, D.; Salvador, P.; Hernández, C.; Calatayud, S.; Esplugues, J.V.; Barrachina, M.D. The flesh ethanolic extract of Hylocereus polyrhizus exerts anti-inflammatory effects and prevents murine colitis. Clin. Nutr. 2016, 35, 1333–1339.
- Perez, G.R.; Vargas, S.R.; Ortiz, H.Y. Wound healing properties of Hylocereus undatus on diabetic rats. Phytother. Res. 2005, 19, 665–668.
- Holanda, M.O. Potencial Terapêutico da Polpa Com Semente da Pitaia Vermelha em Modelo Experimental de Dislipidemia Induzida Por Dieta Hiperlipídica; Embrapa: Fortaleza, Brazil, 2019.
- Yeh, W.J.; Tsai, C.C.; Ko, J.; Yang, H.Y. Hylocereus polyrhizus Peel Extract Retards Alcoholic Liver Disease Progression by Modulating Oxidative Stress and Inflammatory Responses in C57BL/6 Mice. Nutrients 2020, 12, 3884.
- SILVA, F.d.S. Efeito Hipoglicemiante do Extrato Concentrado de Pitaia Vermelha (Hylocereus spp.) em Modelo de Zebrafish (Danio rerio). 2021. Available online: http://www.alice.cnptia.embrapa.br/alice/handle/doc/1143420 (accessed on 20 November 2022).
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469.
- Song, H.; Zheng, Z.; Wu, J.; Lai, J.; Chu, Q.; Zheng, X. White Pitaya (Hylocereus undatus) Juice Attenuates Insulin Resistance and Hepatic Steatosis in Diet-Induced Obese Mice. PLoS ONE 2016, 11, e0149670.
- Hitendraprasad, P.P.; Hegde, K.; Shabaraya, A.R. Hylocereus undatus (Dragon Fruit): A Brief Review. Int. J. Pharm. Sci. Rev. Res. 2020, 60, 55–57.
- Saenjum, C.; Pattananandecha, T.; Nakagawa, K. Antioxidative and anti-inflammatory phytochemicals and related stable paramagnetic species in different parts of dragon fruit. Molecules 2021, 26, 3565.
- Vargas, M.d.L.V.; Cortez, J.A.T.; Duch, E.S.; Lizama, A.P.; Méndez, C.H.H. Extraction and stability of anthocyanins present in the skin of the dragon fruit (Hylocereus undatus). Food Nutr. Sci. 2013, 4, 1221.
- Joshi, M.; Prabhakar, B. Phytoconstituents and pharmaco-therapeutic benefits of pitaya: A wonder fruit. J. Food Biochem. 2020, 44, e13260.
- Namkhah, Z.; Ashtary-Larky, D.; Naeini, F.; Clark, C.C.T.; Asbaghi, O. Does vitamin C supplementation exert profitable effects on serum lipid profile in patients with type 2 diabetes? A systematic review and dose-response meta-analysis. Pharmacol. Res. 2021, 169, 105665.
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497.
- Pires, I.V.; Sakurai, Y.C.N.; Ferreira, N.R.; Moreira, S.G.C.; da Cruz Rodrigues, A.M.; da Silva, L.H.M. Elaboration and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from pitaya. Molecules 2022, 27, 8310.
- Kumorkiewicz-Jamro, A.; Świergosz, T.; Sutor, K.; Spórna-Kucab, A.; Wybraniec, S. Multi-colored shades of betalains: Recent advances in betacyanin chemistry. Nat. Prod. Rep. 2021, 38, 2315–2346.
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335.
- Moo-Huchin, V.M.; Gonzlez-Aguilar, G.A.; Moo-Huchin, M.; Ortiz-V, E. Carotenoid Composition and Antioxidant Activity of Extracts from Tropical Fruits. 2017. Available online: http://cmuir.cmu.ac.th/jspui/handle/6653943832/63885 (accessed on 15 November 2022).
- Jiang, Y.W.; Sun, Z.H.; Tong, W.W.; Yang, K.; Guo, K.Q.; Liu, G.; Pan, A. Dietary Intake and Circulating Concentrations of Carotenoids and Risk of Type 2 Diabetes: A Dose-Response Meta-Analysis of Prospective Observational Studies. Adv. Nutr. 2021, 12, 1723–1733.
- Lim, H.K.; Tan, C.P.; Karim, R.; Ariffin, A.A.; Bakar, J. Chemical composition and DSC thermal properties of two species of Hylocereus cacti seed oil: Hylocereus undatus and Hylocereus polyrhizus. Food Chem. 2010, 119, 1326–1331.
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634.
- Attar, H.; Gündeşli, M.A.; Urün, I.; Kafkas, S.; Kafkas, N.E.; Ercisli, S.; Ge, C.; Mlcek, J.; Adamkova, A. Nutritional Analysis of Red-Purple and White-Fleshed Pitaya (Hylocereus) Species. Molecules 2022, 27, 808.
- Xu, Y.; Tang, G.; Zhang, C.; Wang, N.; Feng, Y. Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress. Molecules 2021, 26, 7115.
- Jiang, H.; Tang, W.; Song, Y.; Jin, W.; Du, Q. Induction of Apoptosis by Metabolites of Rhei Radix et Rhizoma (Da Huang): A Review of the Potential Mechanism in Hepatocellular Carcinoma. Front. Pharmacol. 2022, 13, 806175.
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557.
- Huang, Y.; Brennan, M.A.; Kasapis, S.; Richardson, S.J.; Brennan, C.S. Maturation Process, Nutritional Profile, Bioactivities and Utilisation in Food Products of Red Pitaya Fruits: A Review. Foods 2021, 10, 2862.
- Xu, J.; Wang, W.; Zhao, Y. Phenolic Compounds in Whole Grain Sorghum and Their Health Benefits. Foods 2021, 10, 1921.
- Arivalagan, M.; Karunakaran, G.; Roy, T.K.; Dinsha, M.; Sindhu, B.C.; Shilpashree, V.M.; Satisha, G.C.; Shivashankara, K.S. Biochemical and nutritional characterization of dragon fruit (Hylocereus species). Food Chem. 2021, 353, 129426.
- Nunes, E.N.; de Sousa, A.S.B.; de Lucena, C.M.; Silva, S.D.M.; de Lucena, R.F.P.; Alves, C.A.B.; Alves, R.E. Pitaia (Hylocereus sp.): Uma revisão para o Brasil. Gaia Sci. 2014, 8, 90–98.
- Castro-Enríquez, D.D.; Montaño-Leyva, B.; Del Toro-Sánchez, C.L.; Juárez-Onofre, J.E.; Carvajal-Millán, E.; López-Ahumada, G.A.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A.; Rodríguez-Félix, F. Effect of Ultrafiltration of Pitaya Extract (Stenocereus thurberi) on Its Phytochemical Content, Antioxidant Capacity, and UPLC-DAD-MS Profile. Molecules 2020, 25, 281.
- Ariffin, A.A.; Bakar, J.; Tan, C.P.; Rahman, R.A.; Karim, R.; Loi, C.C. Essential fatty acids of pitaya (dragon fruit) seed oil. Food Chem. 2009, 114, 561–564.
- Jernimo, M.C.; Orsine, J.V.C.; Borges, K.K.; Novaes, M.R.C.G. Chemical and Physical-Chemical Properties, Antioxidant Activity and Fatty Acids Profile of Red Pitaya Grown In Brazil. J. Drug Metab. Toxicol. 2015, 6, 1–6.
- Luu, T.-T.; Le, T.-L.; Huynh, N.; Quintela-Alonso, P. Dragon fruit: A review of health benefits and nutrients and its sustainable development under climate changes in Vietnam. Czech J. Food Sci. 2021, 39, 71–94.
- Jeronimo, M.C. Caracterização Química, Físico-Química, Atividade Antioxidante e Avaliação dos Efeitos Citotóxicos da Pitaia-Vermelha Cultivada no Brasil. 2016. Available online: https://repositorio.unb.br/handle/10482/22448 (accessed on 6 November 2022).
- Akhiruddin, M.A.-S. Nutritional Composition, Antioxidant Properties of Hylocereus Polyrhizus Powder and Their Effects on Plasma Glucose Level and Lipid Profiles in Diabetic Rats and Pre-diabetic Subjects. Ph.D. Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2013.
- Shafie, S.R. Nutritional Composition and Antioxidant Properties of Spray Pitaya Powder (Hylocereus Polyrhizus Briton & Rose) and Its Supplementation Effects on Selected Biomarkers in Normocholestrolemic Subjects. Ph.D. Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2012.
- Harahap, N.S.; Amelia, R. Red dragon fruit (Hylocereus polyrhizus) extract decreases lactic acid level and creatine kinase activity in rats receiving heavy physical exercise. Open Access Maced. J. Med. Sci. 2019, 7, 2232.
- Rusip, G.; Ilyas, S.; Lister, I.N.E.; Ginting, C.N.; Mukti, I. The effect of ingestion of red dragon fruit extract on levels of malondialdehyde and superoxide dismutase after strenuous exercise in rats (Rattus norvegicus). F1000Research 2021, 10, 1061.
- Putri, M.D.; Wiboworini, B.; Dirgahayu, P. Red dragon fruit juice in reducing ros levels and insulin resistance In rats with type 2 diabetes mellitus model. J. Nutr. 2021, 10, 6–14.
- Eldeen, I.M.S.; Foong, S.Y.; Ismail, N.; Wong, K.C. Regulation of pro-inflammatory enzymes by the dragon fruits from Hylocereus undatus (Haworth) and squalene-its major volatile constituents. Pharmacogn. Mag. 2020, 16, 81.
- Amin, M.; Nur, M.; Uddin, R.; Meghla, N.S.; Uddin, M.J. In Vitro Anti-Oxidant, Anti-Inflammatory, Anti-Bacterial, and Cytotoxic Effects of Different Extracted Colorants from Two Species of Dragon Fruit (Hylocereus Spp.); Elsevier: Amsterdam, The Netherlands, 2022.
- Al-Radadi, N.S. Biogenic proficient synthesis of (Au-NPs) via aqueous extract of Red Dragon Pulp and seed oil: Characterization, antioxidant, cytotoxic properties, anti-diabetic anti-inflammatory, anti-Alzheimer and their anti-proliferative potential against cancer cell lines. Saudi J. Biol. Sci. 2022, 29, 2836–2855.
- Wichienchot, S.; Jatupornpipat, M.; Rastall, R. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. 2010, 120, 850–857.
- Dasaesamoh, R.; Youravong, W.; Wichienchot, S. Digestibility, fecal fermentation and anti-cancer of dragon fruit oligosaccharides. Int. Food Res. J. 2016, 23, 2581–2587.
- Temak, Y.; Cholke, P.; Mule, A.; Shingade, A.; Narote, S.; Kagde, A.; Lagad, R.; Sake, V. In vivo and in-vitro evaluation of antimicrobial activity of peel extracts of red dragon fruit (Hylocereus polyrhizus). Res. J. Pharmacogn. Phytochem. 2019, 11, 23–26.
- Sushmitha, H.S.; Roy, C.L.; Gogoi, D.; Velagala, R.D.; Nagarathna, A.; Balasubramanian, S.; Rajadurai, M. Phytochemical and Pharmacological Studies on Hylocereus undatus Seeds: An In Vitro Approach. World J. Pharmacol. Res. 2018, 7, 986–1006.
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136.
- Divakaran, D.; Lakkakula, J.R.; Thakur, M.; Kumawat, M.K.; Srivastava, R. Dragon fruit extract capped gold nanoparticles: Synthesis and their differential cytotoxicity effect on breast cancer cells. Mater. Lett. 2019, 236, 498–502.
- Padmavathy, K.; Kanakarajan, S.; Karthika, S.; Selvaraj, R.; Kamalanathan, A. Phytochemical Profiling And Anticancer Activity Of Dragon Fruit Hylocereus Undatus Extracts Against Human Hepatocellular Carcinoma Cancer (Hepg-2) Cells. Int. J. Pharm. Sci. Res. 2021, 12, 2770–2778.
- Wu, L.C.; Hsu, H.W.; Chen, Y.C.; Chiu, C.C.; Lin, Y.I.; Ho, J.A.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327.
- Khoo, H.E.; He, X.; Tang, Y.; Li, Z.; Li, C.; Zeng, Y.; Tang, J.; Sun, J. Betacyanins and Anthocyanins in Pulp and Peel of Red Pitaya (Hylocereus polyrhizus cv. Jindu), Inhibition of Oxidative Stress, Lipid Reducing, and Cytotoxic Effects. Front. Nutr. 2022, 9, 1390.
- Guimarães, D.A.B.; De Castro, D.; de Oliveira, F.L.; Nogueira, E.M.; da Silva, M.A.M.; Teodoro, A.J. Pitaya Extracts Induce Growth Inhibition and Proapoptotic Effects on Human Cell Lines of Breast Cancer via Downregulation of Estrogen Receptor Gene Expression. Oxid Med. Cell Longev. 2017, 2017, 7865073.
- Abd Hadi, N.; Mohamad, M.; Rohin, M.A.K.; Yusof, R.M.J.B.S. Effects of Red pitaya fruit (Hylocereus polyrhizus) consumption on blood glucose level and lipid profile in type 2 diabetic subjects. Borneo Sci. 2016, 31.
- Poolsup, N.; Suksomboon, N.; Paw, N.J. Effect of dragon fruit on glycemic control in prediabetes and type 2 diabetes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0184577.
- Holanda, M.O.; Lira, S.M.; da Silva, J.Y.G.; Marques, C.G.; Coelho, L.C.; Lima, C.L.S.; Costa, J.T.G.; da Silva, G.S.; Santos, G.B.M.; Zocolo, G.J.; et al. Intake of pitaya (Hylocereus polyrhizus (FAC Weber) Britton & Rose) beneficially affects the cholesterolemic profile of dyslipidemic C57BL/6 mice. Food Biosci. 2021, 42, 101181.
- Fadlilah, S.; Sucipto, A. Dragon Fruit (Hylocereuspolyrhizus) Effectively Reduces Fasting Blood Sugar Levels and Blood Pressure on Excessive Nutritional Status. Pak. J. Med. Health Sci. 2020, 14, 1405–1412.
- Marietta, S.; Budhiarta, A.; Weta, I.W. The supplementation effect of Red Dragon fruit’s skin extract on the fasting blood glucose and lipid profiles in male Wistar rats with diabetes mellitus and dyslipidemia. Neurol. Spinale Medico Chir. 2020, 3, 87–92.
- Setiawan, N.; Shintawati, R.; Priyandoko, D. The role of red dragon fruit peel (Hylocereus polyrhizus) to improvement blood lipid levels of hyperlipidaemia male mice. J. Phys. Conf. Ser. 2018, 1013, 012167.
- Ravichandran, G.; Lakshmanan, D.K.; Murugesan, S.; Elangovan, A.; Rajasekaran, N.S.; Thilagar, S. Attenuation of protein glycation by functional polyphenolics of dragon fruit (Hylocereus polyrhizus); an in vitro and in silico evaluation. Food Res. Int. 2021, 140, 110081.
- Rosa Martha, P.G.; Susana Gabriela, E.A. Ursane derivatives isolated from leaves of Hylocereus undatus inhibit glycation at multiple stages. Chin. J. Nat. Med. 2018, 16, 856–865.
- Sewidan, N.; Abu Khalaf, R.; Mohammad, H.; Hammad, W. In-Vitro Studies on Selected Jordanian Plants as Dipeptidyl Peptidase-IV Inhibitors for Management of Diabetes Mellitus. Iran. J. Pharm. Res. 2020, 19, 95–102.
- Hendra, R.; Khodijah, R.; Putri, R.; Amalia, R.; Haryani, Y.; Teruna, H.Y.; Abdulah, R. Cytotoxicity and Antiplasmodial Properties of Different Hylocereus polyrhizus Peel Extracts. Med. Sci. Monit. Basic Res. 2021, 27, e931118.
- Luo, H.; Cai, Y.; Peng, Z.; Liu, T.; Yang, S. Chemical composition and in vitro evaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel. Chem. Cent J. 2014, 8, 1.
- Shen, L.; Xiong, X.; Zhang, D.; Zekrumah, M.; Hu, Y.; Gu, X.; Wang, C.; Zou, X. Optimization of betacyanins from agricultural by-products using pressurized hot water extraction for antioxidant and in vitro oleic acid-induced steatohepatitis inhibitory activity. J. Food Biochem. 2019, 43, e13044.
- Chang, Y.J.; Pong, L.Y.; Hassan, S.S.; Choo, W.S. Antiviral activity of betacyanins from red pitahaya (Hylocereus polyrhizus) and red spinach (Amaranthus dubius) against dengue virus type 2 (GenBank accession no. MH488959). Access Microbiol. 2020, 2, acmi000073.
- Wahdaningsih, S.; Wahyuono, S.; Riyanto, S.; Murwanti, R. Terpenoid-lupeol of red dragon fruit (Hylocereus polyrhizus) and its immunomodulatory activity. Pak. J. Pharm. Sci. 2020, 33, 505–510.
- Hartono, M.R.; Suardita, K.; Yuliati, A. Proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cell after exposure to red flesh dragon fruit extract. Dent. Res. J. 2020, 17, 107–113.
- Cheok, A.; Xu, Y.; Zhang, Z.; Caton, P.W.; Rodriguez-Mateos, A. Betalain-rich dragon fruit (pitaya) consumption improves vascular function in adult men and women: A double-blind, randomized controlled crossover trial. Am. J. Clin. Nutr. 2021, 115, 1418–1431.
More
