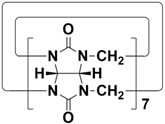
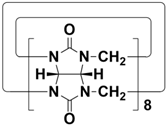
In 2015, Li and co-workers synthesized a new macrocyclic host named biphenarenes, which including basic biphen[n]arenes, functional biphen[n]arenes, and cage compounds. Typically, biphenarenes are made up of 4,4′-biphenol or 4,4′-biphenol ether units linked by methylene bridges at the 3- and 3′-positions. The synthesis of biphenarenes is based on the linking of reaction modules to form macrocycles by Friedel–Crafts alkylation. In addition, modular synthetic strategy is a versatile method for the synthesis of biphenarenes, which can increase the cavity sizes by changing the structural units. For example, the cavity sizes of biphenarenes can be easily increased using long and rigid structural units or increasing the number of structural units. Meanwhile, gram-scale synthesis of biphenarenes is easily achieved in a laboratory. The purification of biphenarenes can be achieved by column chromatography and recrystallization. Furthermore, biphenarenes are easy to prepare since they can be obtained by a one-step condensation reaction using commercial reagents. Biphenarenes show good performance in adsorptive separation, sensing and drug delivery, and have broad application prospects in chemistry, biology, materials science and other fields.
1. Introduction
In the middle of the last century, the discovery of crown ethers laid the foundation for the development of synthetic molecules that could engage in non-covalent interactions
[1]. Then, the concepts of supramolecular chemistry and host–guest chemistry came into being
[2][3][4]. In the development of supramolecular chemistry, the synthesis of novel macrocyclic hosts with special properties is an enduring topic
[5][6][7][8]. Macrocyclic hosts, including crown ethers
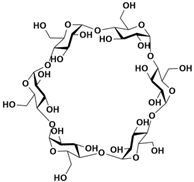
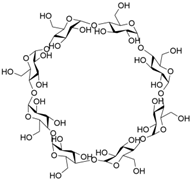
[9][10], cyclodextrins
[11][12][13][14][15], calixarenes
[16], cucurbiturils
[17][18][19], pillararenes
[20][21][22][23][24][25][26], coronarenes
[27] and oxatubarenes
[28], are attractive supramolecular hosts with inherent cavities. Thanks to their excellent host–guest properties, macrocyclic hosts play important roles in the fields of chemistry
[29][30][31][32], materials science
[33][34][35][36], biology
[37][38][39][40][41], etc.
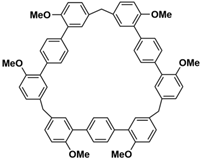
In 2015, Li and co-workers constructed a series of customizable macrocycles named biphenarenes using a modular synthetic strategy
[42]. Since then, the host–guest properties, supramolecular assembly behaviors and functional applications of biphenarenes have been widely explored
[43][44][45][46]. On the one hand, it is easy to obtain biphenarene derivatives containing alkoxy, hydroxy and anionic/cationic groups due to the easy synthesis and derivatization of biphenarenes. The selective complexation of cationic, anionic and neutral guest molecules with biphenarenes can be achieved in different solvents
[47][48][49][50]. On the other hand, biphenarenes with large cavities can be prepared by reasonably adjusting the number of structural units, overcoming the limitation of traditional macrocycles with small cavities (≤10 Å). Customizable cavity sizes make it possible to encapsulate large guests or molecules, which will effectively expand the application range of biphenarenes. The discovery of biphenarenes has greatly enriched the toolbox of synthetic macrocyclic hosts and will also promote the further development of supramolecular chemistry.
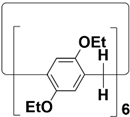
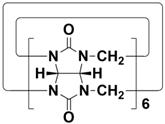
2. Structures of Biphenarenes
Macrocyclic hosts are useful tools for the research of non-covalent interactions
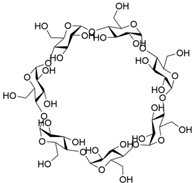
[51][52][53]. Host–guest properties of traditional macrocycles (such as crown ethers, cyclodextrins, calixarenes, cucurbiturils and pillararenes) have been widely studied in the past few decades
[54][55][56][57][58]. However, traditional macrocycles typically have cavity sizes of less than 10 Å (
Table 1), and it is very challenging to prepare giant macrocycles. This makes them generally suitable for binding small- or medium-sized guests, but it is difficult to accommodate large guest molecules. Increasing the number of structural units is a common way to increase the cavity size of macrocycles. However, the simple addition of structural units tends to distort the structures of macrocycles, and it is difficult to obtain macrocycles with large cavities. This severely limits the further applications of macrocyclic hosts. In addition, for traditional macrocycles, functional groups are usually introduced through their portals rather than the skeleton.
In order to solve these problems, many macrocyclic compounds with large, rigid cavities have been developed. For example, cycloparaphenylenes, consisting of a simple string of benzene, have attracted scientists because of their simple and beautiful structure and potential applications in materials science and supramolecular chemistry
[59][60]. In 2015, Li and co-workers synthesized a new macrocyclic host named biphenarenes, which including basic biphen[
n]arenes, functional biphen[
n]arenes, and cage compounds
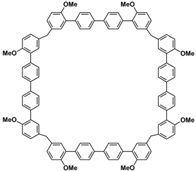
[42]. Typically, biphenarenes are made up of 4,4′-biphenol or 4,4′-biphenol ether units linked by methylene bridges at the 3- and 3′-positions. The synthesis of biphenarenes is based on the linking of reaction modules to form macrocycles by Friedel–Crafts alkylation. In addition, modular synthetic strategy is a versatile method for the synthesis of biphenarenes, which can increase the cavity sizes by changing the structural units (
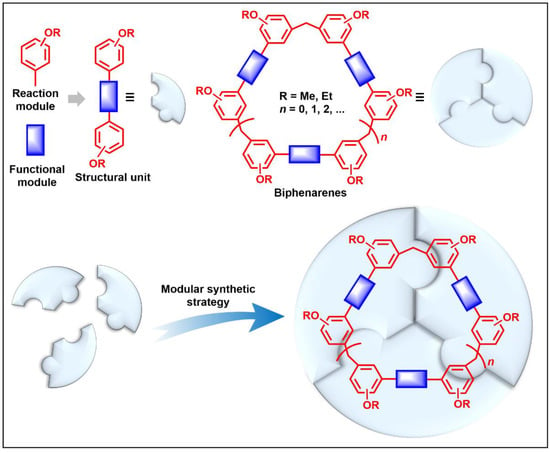
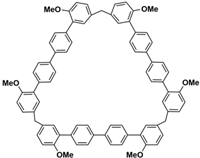
Figure 1)
[48][49]. For example, the cavity sizes of biphenarenes can be easily increased using long and rigid structural units or increasing the number of structural units. Meanwhile, gram-scale synthesis of biphenarenes is easily achieved in a laboratory. The purification of biphenarenes can be achieved by column chromatography and recrystallization. Furthermore, biphenarenes are easy to prepare since they can be obtained by a one-step condensation reaction using commercial reagents. Biphenarenes show good performance in adsorptive separation, sensing and drug delivery, and have broad application prospects in chemistry, biology, materials science and other fields.
Figure 1.
Modular synthetic strategy of biphenarenes.
Compared with traditional macrocycles, the structures of biphenarenes have two advantages:
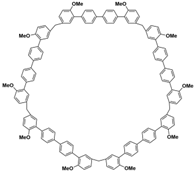
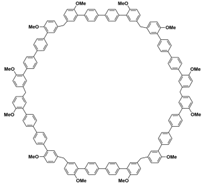
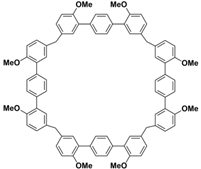
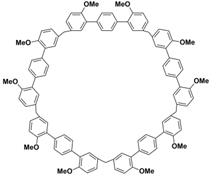
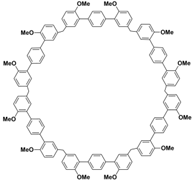
(i) Large cavity. Like many other structurally related macrocyclic hosts, biphenarenes are formed from suitable electron-rich aromatic building blocks and formaldehyde by repeated Friedel–Crafts alkylation. The normal strategy to increase cavity size involves increasing the number of subunits along the ring, but this can concomitantly lead to an increase in conformational flexibility accompanied by a collapse of the cavity. In contrast, the cavity size of biphenarenes is increased by incorporating spacers (or functional modules) between the terminal aromatic units of the building blocks, making macrocycles with large cavity easily accessible. Because the structural units of biphenarenes are independent of each other, the cavity sizes can be easily expanded using long and rigid structural units without affecting the cyclization reaction. For example, Li and co-workers synthesized macrocyclic hosts of terphen[
n]arenes (TP
ns,
n = 3–6) and quaterphen[
n]arenes (QP
ns,
n = 3–6)
[61]. TP
ns and QP
ns have larger cavities and better self-assembly properties compared to traditional macrocycles because of their longer and more rigid structural units (
Table 2). Among them, the largest macrocyclic molecule QP6 has a cavity size of more than 30 Å, which is much larger than that of classic macrocyclic hosts. The customizable cavity sizes of biphenarenes facilitate the encapsulation of large guest molecules (such as polypeptides or other biomacromolecules) and effectively enrich their host–guest properties. Due to these advantages, biphenarenes have potential applications in the field of supramolecular self-assembly.
Table 1.
Chemical structures and diameters of traditional macrocyclic hosts.
Table 2.
Chemical structures and diameters of typical biphenarenes.
(ii) Easy functionalization. Furthermore, the development of new applications of macrocyclic hosts is inseparable from the functionalization of macrocycles
[62][63][64]. Whereas the skeletons of most macrocycles cannot be changed, their functional substituents can be introduced on their portals
[65][66][67]. The synthesis of biphenarenes is achieved through modular synthetic strategy, and the functionalization can not only introduce functional substituents on their portals, but also realize functionalization by changing the functional modules. By changing the functional modules of biphenarenes by modular synthesis, endo-functionalized macrocycles can be easily synthesized
[47]. This method not only expands the cavity sizes of biphenarenes, but also enriches their host–guest properties and functions. In this way, phenyl, naphthyl, benzofuranyl, benzothiophyl and even π-electron-rich donors (e.g., anthryl, pyrenyl, azophenyl) can also be introduced into the macrocyclic backbones of biphenarenes.
The structural properties of biphenarenes make the synthesis of functional macrocycles more economical and efficient. It is possible to integrate different functional building units into a macrocycle, greatly expanding the toolbox of biphenarenes. The diversity of structures and functions of biphenarenes show extensive binding abilities and extraordinary self-assembly behaviors, laying the foundation for their vigorous development in the field of supramolecular chemistry.
 [56
[56