Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Alice Vilela.
Phenolic compounds are classified as primary antioxidants and originate from one of the main classes of secondary metabolites in plants. They have antioxidant properties through several mechanisms: (i) the ability to remove free radicals and inhibit the formation of reactive species during the normal course of metabolism; (ii) preventing the occurrence of damage to lipids, proteins, and nucleic acids; and (iii) preventing consequent cell damage and death. Thus, they are commonly associated with preventing the development of cardiovascular diseases, neurodegenerative diseases, autoimmune diseases, diabetes, and cancer.
- nutraceutical properties
- human health
- phenolic compounds
1. Natural Phenolic Antioxidants in Beverages
Natural antioxidants can be obtained from plants (fruits, legumes, and vegetables), mushrooms, and algae, and are classified as phenolic compounds, vitamins, and carotenoids [65,66,67][1][2][3].
In recent years, there has been a growing preference for natural phenolic antioxidants over artificial ones, fundamentally due to the increasing demand by consumers for functional foods and beverages with the addition of natural additives, which maintain their nutritional properties and flavor [68][4]. Besides, this trend results, according to the same authors, from the preference currently given to natural phenolic antioxidants in food stabilization, and the restrictions applied by the responsible entities on the use of synthetic phenolic antioxidants [69,70][5][6].
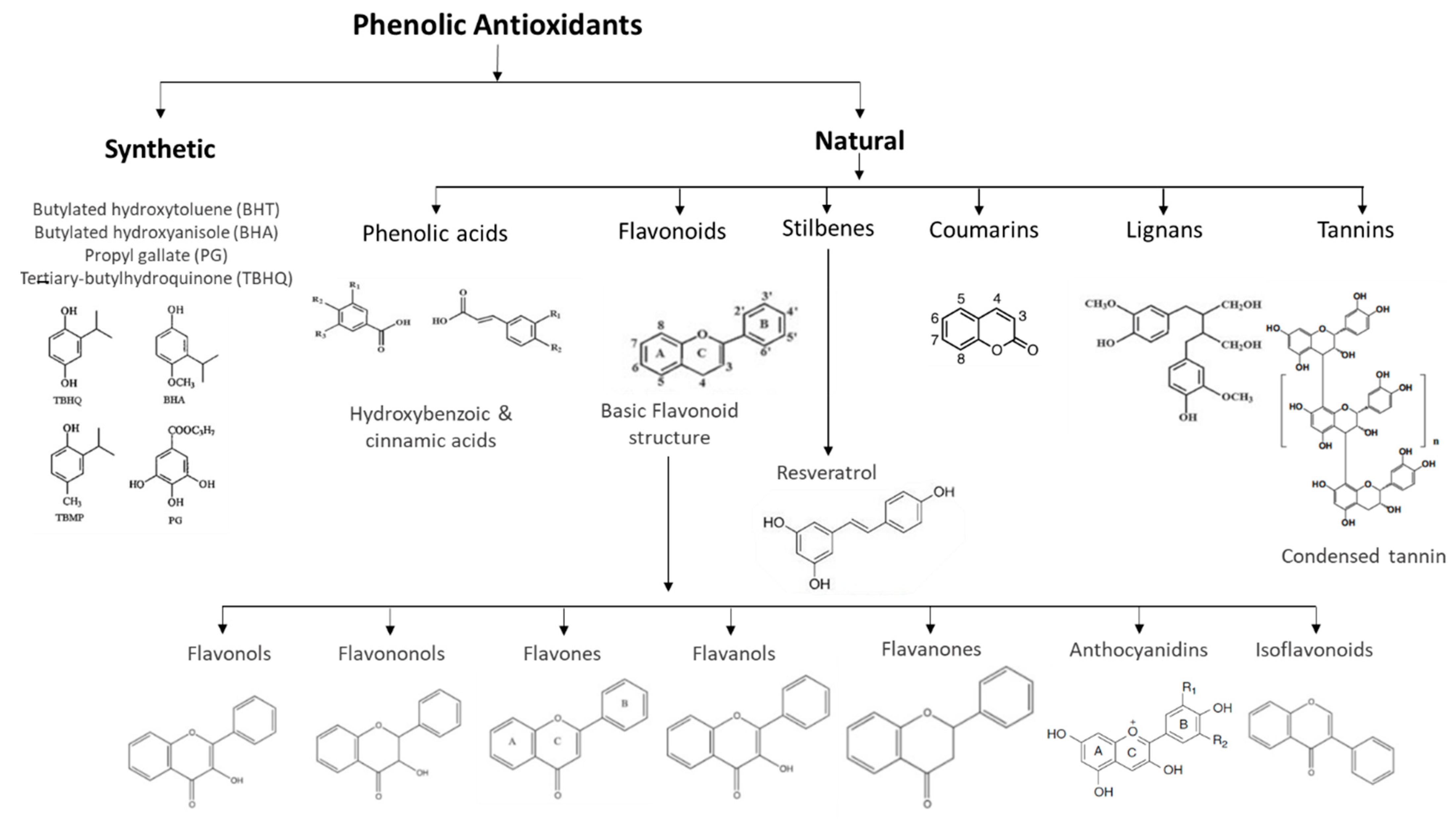
Natural phenolic antioxidants have a great diversity of structures, in which the basic monomer of polyphenols is the phenolic ring [71][7]. They are divided into several classes, the most representative of which are flavonoids and phenolic acids. For their part, flavonoids are further divided into flavones, flavanones, flavonols, flavanols, isoflavones, and phenolic acids and are generally classified into hydroxybenzoic and hydroxycinnamic acids [72][8].
Stilbenes are a group of phenolic compounds that share a similar chemical structure to flavonoids (Figure 61). Trans-resveratrol is one of the most recognized stilbenes, present mostly in glycosylated forms. Red wine, as well as the red grapes that originate this fermented drink, are rich in resveratrol. Several studies show that moderate consumption of red wine leads to a reduction in the development of cardiovascular diseases and atherosclerotic plaques, and provides neuroprotective, antidiabetic, anti-inflammatory, antioxidant, anticarcinogenic, and antiviral activity [73,74,75][9][10][11].
When analyzing different types of wines, namely, whites, rosés, and reds, Paixão et al. [76][13] showed that red wine had significantly higher phenolic levels than the rosé and white wines, and consequently exhibited the highest antioxidant power. According to Fiori et al. [77][14], the highest concentration of resveratrol is higher in red wine than in white wine because it is present in the skin and seeds of grapes. Thus, the different phenolic composition of wines is related to the grape variety, as well as the edaphoclimatic conditions of the region where the grapes are produced, cultural practices, the stage of ripeness [78[15][16],79], maceration [80[17][18],81], yeasts used in the vinification process [82][19], and other winemaking conditions [83][20].
Cordova and Sumpio [84][21] also concluded that red wine has more health-promoting activity than beer or spirits due to its richer content of phenolic compounds, hence the increased interest in the nutraceutical value of wines.
Additionally, herbal teas and infusions are rich in natural antioxidants, mainly flavonoids: theaflavins, bis-flavanols, and fulvic acids. Their consumption has increased because they are recognized as having anticariogenic properties and antimicrobial and anticancer activity [85,86][22][23]. McCarthy et al. [87][24] studied the antioxidant potential of plant extracts and compared them with synthetic antioxidants and vitamin E, incorporating them in pork. The catechins present in tea were shown to be more effective in terms of their antioxidant power, compared to butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), and antioxidant activity was evaluated through the thiobarbituric acid reactive substance assay (TBARS assay—reactive species of thiobarbituric acid).
The market for natural antioxidants is growing and new products have appeared on the market with a healthy image, namely, smoothies, functional drinks, and yogurts with green tea, grape seed, lemon balm, and aloe vera, among others [68][4].
It is important to note that there are natural antioxidants that have lower antioxidant activity than their synthetic equivalents, which can lead to the use of a higher dosage, causing toxicity reactions [88][25]. Thus, the intensification of toxicity studies of these compounds is necessary to know the limits of their use.
2. Synthetic Phenolic Antioxidants in Beverages
Several synthetic phenolic antioxidants (SPAs) are widely used in industrial and commercial products, but only a few can legally be added to food products [89][26]. Thus, with a common molecular structure in which the phenolic rings are replaced by alkyls hindered in the ortho position [70][6], butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate (PG), and tertiary-butylhydroquinone (TBHQ) are commonly used as food antioxidants [90][27]. However, BHA and BHT are the antioxidants most used as additives in food products [91,92][28][29].
The poor stability of natural antioxidants has increased the number of SPAs that are preferred for use in food and beverages to prevent and delay lipid oxidation reactions, preventing the formation of foreign flavors and undesirable chemical compounds, such as aldehydes, ketones, and organic acids, and prolong the shelf life of products [93][30].
SPAs can be used alone or in combination. Thus, BHA is commonly found in dry cereals, derived from potatoes, in cooked foods (boiled or fried and desserts), and in drinks [90,94][27][31]. In food supplements, condiments, spices, chewing gum, and oils, BHT can be used alone or in combination with BHA or TBHQ [95][32]. As a preservative, TBHQ is used in edible animal fats, meat products, and unsaturated vegetable oils [96][33]. Propyl gallate has been used in the food industry as a stabilizer in fatty foods and as an additive in mayonnaise, fats, edible fats, and baked goods [97][34].
The presence of a wide range of undesirable compounds in foods and beverages has made the area of food safety increasingly relevant, to provide the population with the necessary high-quality food. Therefore, the growing concern about using safe and environmentally friendly food products has recently led to the realization of several studies to find out if the use of SPAs in food and beverages is safe for health. Their conclusions are contradictory. In some studies, SPAs revealed antimutagenic and antitumor properties [98,99,100,101,102][35][36][37][38][39] but, in others, allergic reactions, including asthma and hives [103][40], toxic effects in some animal tissues [104][41], liver toxicity, endocrine-disrupting effects, and even carcinogenicity [93,105,106,107][30][42][43][44] were reported, questioning their use [70,108][6][45].
This issue generated concern on the part of the governments of the European Union and most countries to create legislation that regulates the use of SPAs, either individually or in mixtures, as the market for combined natural or synthetic phenolic antioxidants is expected to have a growth rate of around 5% by 2023 [109][46]. In the European Union, the use of certain SPAs has been restricted and has even been banned in soft drinks [89][26].
References
- Sikora, E.; Cieślik, E.; Topolska, K. The sources of natural antioxidants. Acta Sci. Pol. Technol. Aliment. 2008, 7, 5–17.
- Jayaprakasha, G.; Singh, R.; Sakariah, K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290.
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399.
- Massini, L.; Rico, D.; Martín-Diana, A.B.; Barry-Ryan, C. Quality Markers of Functional Tomato Juice with Added Apple Phenolic Antioxidants. Beverages 2016, 2, 4.
- Berdahl, D.R.; Nahas, R.I.; Barren, J.P. Synthetic, and natural antioxidant additives in food stabilization: Current applications and future research. In Oxidation in Foods and Beverages and Antioxidant Applications; Decker, E.A., Elias, R.J., McClements, D.J., Eds.; Woodhead Publishing: Cambridge, UK, 2010; Volume 1, pp. 272–313.
- Liu, R.; Mabury, S.A. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719.
- Dragovicuzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The Content of Polyphenols and Carotenoids in Three Apricot Cultivars Depending on Stage of Maturity and Geographical Region. Food Chem. 2007, 102, 966–975.
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699.
- Fernández-Mar, M.I.; Mateos, R.; Garcıa-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813.
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell Longev. 2015, 837042.
- Gea, A.; Sánchez-Tainta, A. Red Wine Moderate Consumption and at Mealtimes. In Prevention of Cardiovascular Disease through the Mediterranean Diet; Sánchez-Villegas, A., Sánchez-Tainta, A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 151–157.
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897.
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214.
- Fiori, L.; de Faveri, D.; Casazza, A.A.; Perego, P. Grape by-products: Extraction of polyphenolic compounds using supercritical CO2 and liquid organic solvent—A preliminary investigation Subproductos de la uva: Extraccion de compuestos polifenolicos usando CO2 supercritico y disolventes organicos liquidos—Una investigacion preliminar. CyTA J. Food 2009, 7, 163–171.
- Obreque-Slier, E.; Peńa-Neira, A.; López-Solís, R.; Zamora-Marín, F.; Ricardo-da Silva, J.M.; Laureano, O. Comparative study of the phenolic composition of seeds and skins from Carménčre and Cabernet Sauvignon grape varieties (Vitis vinifera L.) during ripening. J. Agric Food Chem. 2010, 58, 3591–3599.
- Giuffrč, A.M. HPLC-DAD detection of changes in phenol content of red berry skins during grape ripening. Eur. Food Res. Technol. 2013, 237, 555–564.
- Budić-Leto, I.; Lovrić, T.; Pezo, I.; Gajdoš Kljusurić, J. Study of dynamics of polyphenol extraction during traditional and advanced maceration processes of the Babić grape variety. Food Technol. Biotechnol. 2005, 43, 47–53.
- Klenar, I.; Berović, M.; Wondra, M. Phenolic compounds from the fermentation of cultivars Cabernet Sauvignon and Merlot from the Slovenian coastal region. Food Technol. Biotechnol. 2004, 42, 11–17.
- Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46.
- Coletta, A.; Berto, S.; Crupi, P.; Cravero, M.C.; Tamborra, P.; Antonacci, D.; Daniele, P.G.; Prenesti, E. Effect of viticulture practices on concentration of polyphenolic compounds and total antioxidant capacity of Southern Italy red wines. Food Chem. 2014, 152, 467–474.
- Cordova, A.C.; Sumpio, B.E. Polyphenols are medicine: Is it time to prescribe red wine for our patients? Int. J. Angiol. 2009, 18, 111–117.
- Soni, R.P.; Katoch, M.; Kumar, A.; Ladohiya, R.; Verma, P. Tea: Production, Composition, Consumption and it’s Potential as an Antioxidant and Antimicrobial Agent. Int. J. Food. Ferment. Technol. 2015, 5, 95–106.
- Vilela, A.; Pinto, T. Grape Infusions: The Flavor of Grapes and Health-Promoting Compounds in Your Tea Cup. Beverages 2019, 5, 48.
- McCarthy, T.L.; Kerry, J.P.; Kerry, J.F.; Lynch, P.B.; Buckley, D.J. Evaluation of the antioxidant potential of natural food/plant extracts as compared with synthetic antioxidants and vitamin E in raw and cooked pork patties. Meat Sci. 2001, 58, 45–52.
- Bártíková, H.; Skálová, L.; Valentova, K.; Matoušková, P.; Szotáková, B.; Martin, J.; Kvita, V.; Boušová, I. Efect of oral administration of green tea extract in various dosage schemes on oxidative stress status of mice in vivo. Acta Pharm. 2015, 65, 65–73.
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Determination of synthetic phenolic antioxidants in soft drinks by stir-bar sorptive extraction coupled to gas chromatography-mass spectrometry. Food Addit. Contam. Part A 2015, 32, 665–673.
- Makahleh, A.; Saad, B.; Bari, M.F. Synthetic phenolics as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2015; pp. 51–78.
- Furia, T.E. CRC Handbook of Food Additives, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1980; Volume 2, p. 424. ISBN 0849305438.
- Nieva-Echevarría, B.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. 2,6-Di-tert-butyl-hydroxytoluene and its metabolites in foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 67–80.
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT. Int. J. Toxicol. 2002, 21 (Suppl. 2), 19–94.
- Dolatabadi, J.E.N.; Kashanian, S. A review on DNA interaction with synthetic phenolic food additives. Food Res. Int. 2010, 43, 1223–1230.
- Lavagnini, I.; Urbani, A.; Magno, F. Overall calibration procedure via a statistically based matrix-comprehensive approach in the stir-bar sorptive extraction-thermal desorption-gas chromatography-mass spectrometry analysis of pesticide residues in fruit-based soft drinks. Talanta 2011, 83, 1754–1762.
- Kashanian, S.; Dolatabadi, J.E.N. DNA binding studies of 2-tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2009, 116, 743–747.
- Zurita, J.L.; Jos, A.; Peso, A.D.; Salguero, M.; López-Artíguez, M.; Repetto, G. Ecotoxicological effects of the antioxidant additive propyl gallate in five aquatic systems. Water Res. 2007, 41, 2599–2611.
- Iverson, F. In vivo studies on butylated hydroxyanisole. Food Chem. Toxicol. 1999, 37, 993–997.
- Botterweck, A.A.M.; Verhagen, H.; Goldbohm, R.A.; Kleinjans, J.; van den Brandt, P.A. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: Results from analyses in the Netherlands Cohort Study. Food Chem. Toxicol. 2000, 38, 599–605.
- Singh, B.; Mense, S.M.; Remotti, F.; Liu, X.; Bhat, H.K. Antioxidant butylated hydroxyanisole inhibits estrogen-induced breast carcinogenesis in female ACI rats. J. Biochem. Mol. Toxicol. 2009, 23, 202–211.
- Zhang, Y.; Choksi, S.; Liu, Z.-G. Butylated hydroxyanisole blocks the occurrence of tumor-associated macrophages in tobacco smoke carcinogen-induced lung tumorigenesis. Cancers 2013, 5, 1643–1654.
- Liang, X.; Tang, Y.; Duan, L.; Cheng, S.; Luo, L.; Cao, X.; Tu, B. Adverse effect of sub-chronic exposure to benzo(a)pyrene and protective effect of butylated hydroxyanisole on learning and memory ability in male Sprague-Dawley rat. J. Toxicol. Sci. 2014, 39, 739–748.
- Simon, R.A. Adverse reactions to food additives. Curr. Allergy Asthma Rep. 2003, 3, 62–66.
- Horváthová, E.; Slameňová, D.; Bonatti, S.; Abbondandolo, A. Reduction of genotoxic effects of MNNG by butylated hydroxyanisole. Neoplasma 1999, 46, 356–362.
- Yang, X.; Song, W.; Liu, N.; Sun, Z.; Liu, R.; Liu, Q.S.; Zhou, Q.; Jiang, G. Synthetic phenolic antioxidants cause perturbation in steroidogenesis in vitro and in vivo. Environ. Sci. Technol. 2018, 52, 850–858.
- Dassarma, B.; Nandi, D.K.; Gangopadhyay, S.; Samanta, S. Hepatoprotective effect of food preservatives (butylated hydroxyanisole, butylated hydroxytoluene) on carbon tetrachloride-induced hepatotoxicity in rat. Toxicol. Rep. 2018, 5, 31–37.
- Pérez-Albaladejo, E.; Lacorte, S.; Porte, C. Differential toxicity of alkylphenols in JEG-3 human placental cells: Alteration of P450 aromatase and cell lipid composition. Toxicol. Sci. 2019, 167, 336–346.
- Valentao, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidative properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical, and hypochlorous acid. J. Agric. Food Chem. 2002, 50, 4989–4993.
- Phenolic Antioxidant Market Research Report–Forecast to 2023. Market Research Future. Available online: https://www.marketresearchfuture.com/reports/phenolic-antioxidant-market-3937#answer1 (accessed on 13 December 2020).
More
