Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Dongsoo Yang and Version 2 by Conner Chen.
Polyketides are a diverse set of natural products with versatile applications as pharmaceuticals, nutraceuticals, and cosmetics, to name a few. Of several types of polyketides, aromatic polyketides comprising type II and III polyketides contain many chemicals important for human health such as antibiotics and anticancer agents. Most aromatic polyketides are produced from soil bacteria or plants, which are difficult to engineer and grow slowly in industrial settings.
- aromatic polyketide
- enzyme engineering
- metabolic engineering
1. Introduction
Polyketides are a diverse set of natural products which are widely known for their medicinal activities such as antibiotic, antifungal, antiviral, anticancer, and anti-immunosuppressant activities [1]. Polyketides, comprised of carbon skeletons with β-keto groups with various degrees of reduction, are produced by a series of Claisen condensation reactions of short-chain acyl-CoA molecules such as acetyl-CoA or malonyl-CoA. Enzymes involved in the biosynthesis of polyketide main carbon skeletons are collectively called polyketide synthases (PKSs). After the biosynthesis of the main carbon skeletons, they can be modified by reduction, cyclization, aromatization, and other tailoring reactions (e.g., oxygenation, methylation, and glycosylation), leading to the production of diverse polyketide products. The biosynthetic mechanisms of PKSs are important in that such differences characterize the different types of PKSs and their products.
Depending on the biosynthetic mechanisms, PKSs can be largely classified into three types (I, II, and III) [1]. Among the three types, type I PKSs are again classified into modular type I PKS and iterative type I PKS. Modular type I PKSs are mostly megadalton-sized enzymes comprised of several modules (which are again comprised of several enzymatic domains) responsible for each carbon chain elongation step. A representative example of type I polyketide is erythromycin, an antibiotic, which has been successfully produced by engineered Escherichia coli [2]. There also have been other examples of successful production of type I polyketides including epothilone [3] and a rifamycin precursor [4] by engineered E. coli. Iterative type I PKS, on the other hand, consists of smaller enzyme clusters of which a single assembly line is iteratively utilized for the synthesis of the main carbon skeleton [5]. Representative examples of polyketides produced by iterative type I PKSs are lovastatin (used to treat cardiovascular diseases) [6] and simvastatin (a semisynthetic chemical used to treat high cholesterol), which have been successfully produced by engineered yeast [7].
In contrast to type I PKS, type II PKS refers to a group of discrete enzymes which cooperatively synthesize the main carbon skeleton. The three essential enzymes responsible for the carbon chain biosynthesis are termed the minimal PKS, which comprises ketosynthase α (KSα), ketosynthase β (KSβ), and acyl-carrier protein (ACP). The KSα and the KSβ subunits form a heterodimer which is responsible for the carbon chain elongation. The two subunits form an amphipathic channel at their interface which plays an important role in the determination of the carbon chain length and initial cyclization reactions. In particular, as the KSβ subunit is primarily responsible for determining the carbon chain length, which is directly relevant to the number of malonyl-CoA molecules incorporated, it is also termed the ‘chain length factor (CLF)’ [8]. The biosynthetic mechanism of type III PKS is simpler than that of type II PKS in that it only requires a single ketosynthase, which forms a homodimer, and that it utilizes malonyl-CoA instead of malonyl-ACP. In addition, unlike type II PKS which uses short-chain aliphatic CoAs (e.g., acetyl-CoA, propionyl-CoA) as starter units, a number of type III PKSs accept aromatic CoAs (e.g., coumaroyl-CoA) as starter units [8]. Therefore, many type III PKSs are also part of the chalcone synthase (CHS) family. Since the products of type II and type III PKSs often contain multiple aromatic rings, they are also termed aromatic PKSs. Aromatic polyketides include many chemicals important for human health such as antibiotics (e.g., oxytetracycline, actinorhodin) and anticancer agents (e.g., doxorubicin) [9].
Most polyketides are produced from soil bacteria such as Streptomyces species or plants (mostly type III polyketides). Although these hosts are remarkable organisms with abilities to produce a diverse set of polyketides, the following difficulties hinder such hosts from being used in either laboratory or industrial settings. Streptomyces species are spore-forming and slow-growing bacteria, which are notoriously difficult to engineer due to the limited availability of genetic engineering toolkits [10][11][10,11]. In addition, Streptomyces species show complex gene expression profiles according to different culture conditions due to the existence of diverse sigma factors and complex gene regulatory systems. On top of this, their culture conditions are very complex and the high-cell-density culture of these bacteria is not amenable, making it difficult to achieve high productivity and titer. In addition, many ‘undomesticated’ Streptomyces species still cannot be cultured in laboratory conditions. Even in easily cultivable Streptomyces species, a significant number of cryptic biosynthetic gene clusters (BGCs) that are not expressed unless in particular culture conditions make the problem worse. Due to poorly developed engineering tools, commercially utilized Streptomyces strains producing polyketides have been mostly developed by random mutagenesis. Culturing plants is also problematic since the cultivation often takes several months, in contrast to the time it takes for bacterial culture which is often several days. Additionally, the land and labor required for plant culture largely exceed those of bacterial culture, making it uneconomical. Therefore, efficient production of valuable polyketides by model microorganisms such as E. coli and Saccharomyces cerevisiae with well-established engineering tools is required to achieve optimal titer, productivity, and yield [12]. These model microorganisms are also capable of high-cell-density culture and have well-curated genome-scale metabolic models. Because engineering these hosts is highly amenable, they are also useful for the derivatization of polyketides, enabling the production of ‘unnatural’ polyketides through heterologous expression of diverse tailoring enzymes. Represented by the complete production of erythromycin in E. coli, type I polyketides have been readily produced from engineered E. coli strains [2][13][2,13]. However, heterologous production of aromatic polyketides from model microorganisms had been hampered until recently due to difficulties in the functional expression of type II and type III PKSs in heterologous hosts. The recent development of metabolic engineering and synthetic biology approaches have enabled facile production of several aromatic polyketides in model microorganisms, providing an avenue for the construction of efficient microbial cell factories for the production of diverse and useful aromatic polyketides [14][15][14,15].
2. Strategies for the Heterologous Production of Type II Aromatic Polyketides
Due to difficulties in engineering the native aromatic polyketide producers (mainly the Streptomyces species), as described earlier in the introduction, heterologous production of aromatic polyketides in model microorganisms such as E. coli or S. cerevisiae has been sought. During the last two decades, many researchers have delved into the production of aromatic polyketides by engineered E. coli, as it is one of the best-studied organisms with many characteristics favorable for industrial applications [16][30]. Such characteristics include fast growth, capability for high-cell-density culture, and well-established engineering tools [15]. However, attempts to produce type II polyketides in E. coli had mostly failed due to the highly insoluble and inactive type II minimal PKSs which were observed primarily as inclusion bodies [17]. Cloning of large-sized BGCs with the size of several tens of kilobase pairs with high guanine-cytosine (GC) contents was also problematic.
One of the first studies that enabled bypassing the minimal PKS solubility problem was achieved by engineering a fungal PKS (Figure 1A) [18][16]. In this study, PKS4 (an iterative type I PKS) from Gibberella fujikuroi was dissected and the KS, malonyl-CoA:ACP transferase (MAT), and ACP domains were reassembled to form a synthetic PKS that could mimic a type II PKS [19][28]. The engineered fungal PKS exhibited cyclization regioselectivity that had not been observed from a fungal PKS but rather from a bacterial type II PKS. Introduction of bacterial tailoring enzymes in addition to the synthetic minimal PKS resulted in the production of C18 polyketides PK8 and SEK26 (3 mg/L by fed-batch culture) [19][28]. A new nonaketide (produced from nine CoA units) nonaSEK4 could also be produced by the different combinations of tailoring enzymes, proving the versatility of employing E. coli as a host for PKS engineering. However, such a strategy could only be applied to a limited number of aromatic polyketides and the efficiency of the synthetic PKS was still very low. In another study, one of the major bottlenecks identified for aromatic polyketide biosynthesis was the extremely low expression level of a gene (oxyB) encoding the CLF (Figure 1B) [20][26]. Overexpression of an alternative sigma factor σ54 led to enhanced oxyB transcript level, thus facilitating the functional expression of the minimal PKS, resulting in 2 mg/L of oxytetracycline production in an E. coli strain harboring the oxytetracycline biosynthetic genes. However, the minimal PKS solubility issue was still not resolved by applying such strategies.
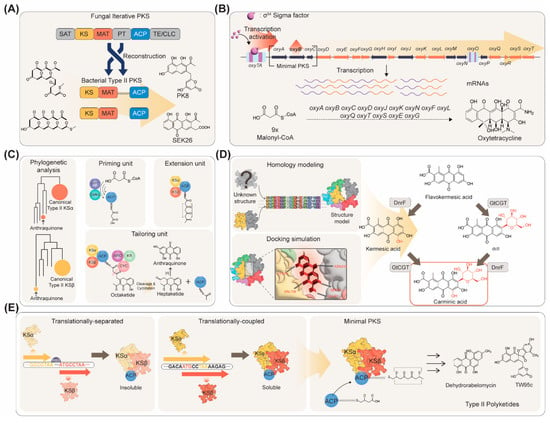
Figure 1. Minimal PKS engineering strategies for the production of aromatic polyketides. (A) Reconstruction of a fungal type II PKS into a synthetic PKS in bacteria for producing aromatic polyketides. (B) Overexpression of sigma factor σ54 to enhance oxyB transcript level, followed by increased production of oxytetracycline in E. coli. (C) Phylogenetic analysis-assisted plug-and-play modes of minimal PKS construction for aromatic polyketide production. (D) Combinatorial application of homology modeling and docking simulation of bottleneck enzymes to construct a novel carminic acid biosynthetic pathway in E. coli. (E) Translational-coupling of alpA and alpB genes to produce soluble forms of minimal PKS followed by increased production of C20 and C24 polyketides.
Recently, a pioneering study led by Takano and colleagues reported the discovery of a minimal PKS from the Gram-negative bacterium Photorhabdus luminescens TT01, which was soluble in E. coli [21][19]. Using the MiBiG (Minimum Information about a Biosynthetic Gene Cluster) repository [22][31], type II minimal PKS candidates were selected (Figure 1C). Then, minimal PKSs that are phylogenetically close to E. coli FabF were identified, which are more likely to be functional in E. coli when introduced. The minimal PKS comprised of AntD (KS), AntE (CLF), and AntF (ACP) from P. luminescens enabled efficient production of an octaketide (produced from eight CoA units) carbon skeleton required for the complete biosynthesis of anthraquinones. Using plug-and-play modes of production by the introduction of various combinations of tailoring enzymes, in addition to the minimal PKS, 1,3,7-trihydroxyanthracene-9,10-dione (AQ-256) could be produced, along with non-natural aromatic polyketides, neomedicamycin, and neochaetomycin [22][31].
Employing the same minimal PKS from P. luminescens, a natural red colorant carminic acid could be produced by metabolically engineered E. coli (Figure 1D) [23][24]. Introduction of P. luminescens antDEF (encoding the type II minimal PKS) along with antB [encoding a phosphopantetheinyl transferase (PPTase)] and antG (encoding a CoA ligase) allowed efficient biosynthesis of the C16 carbon skeleton. Additional introduction of zhuI and zhuJ, both encoding cyclases, from Streptomyces sp. R1128 resulted in the production of a carminic acid precursor, flavokermesic acid, with the titer as high as 180.3 mg/L. However, since the enzymes responsible for the remaining steps (monooxygenation and C-glycosylation) towards carminic acid production have not yet been discovered or were not functional in heterologous microorganisms, biochemical reaction analysis was performed to select enzymes that were expected to perform the desired reactions. DnrF from Streptomyces peucetius was selected as the monooxygenase and GtCGT from Gentiana triflora was selected as the C-glycosyltransferase [23][24]. As the native substrates of these enzymes were not flavokermesic acid, their activities were not enough for carminic acid biosynthesis. To enhance the activities of these enzymes, homology modeling was first performed to predict the 3D structures of the enzymes. Then, docking simulation-assisted mutagenesis of the enzymes was performed to identify enzyme variants with enhanced activities. Such strategies led to the production of carminic acid directly from glucose [23][24]. The GtCGT variant developed in this study (GtCGTV93Q/Y193F) was also capable of C-glycosylating other aromatic polyketides as well, as tested for the production of aloesin, a skin-whitening agent found from Aloe vera.
Another recent breakthrough was made by Jiang and colleagues by the discovery of a minimal PKS that enabled efficient production of C20 and C24 aromatic polyketides in E. coli (Figure 1E) [24][21]. In this study, the alpA and alpB genes from Streptomyces ambofaciens, each encoding KS and CLF, capable of biosynthesis of C20 carbon skeleton were discovered. Interestingly, the two genes were translationally coupled, which is probably one of the reasons for the soluble expression of the AlpA (KS) and AlpB (CLF) enzymes in E. coli. However, the exact mechanism of how translational coupling contributed to the soluble expression of the minimal PKS is not clear. Co-expression of the GroES/EL chaperone further enhanced the solubility of the minimal PKS. One problem that still remained was that the PPTase responsible for the activation of AlpC (ACP) was unknown. Therefore, an alternative ACP, RavC from Streptomyces ravidus, was employed. RavC could be readily activated by Bacillus subtilis Sfp (PPTase), resulting in the successful production of a decaketide (produced from 10 CoA units) dehydrorabelomycin (25 mg/L) [24][21]. It was also reported that additional introduction of AlpS, a thioesterase, could notably increase the efficiency of the biosynthesis of decaketide carbon skeletons, resulting in 0.5 g/L of dehydrorabelomycin production [25][22]. The research team also showed that employing the minimal PKS whiE-ORFIII, -ORFIV, -ORFV from Streptomyces coelicolor resulted in the production of a dodecaketide (produced from 12 CoA units) TW95c [24][21].
The elucidation of three-dimensional structures of type II PKSs can offer valuable insights into the biosynthetic mechanisms of aromatic polyketides and can guide potential engineering strategies. For instance, the recent discovery of the octaketide synthase minimal PKS complex from P. luminescens indicated that a hexaketide is covalently linked to the heterodimer with the ACP [26][32]. Such observations provide crucial insights for engineering type II PKSs, either to enhance biosynthetic efficiency or to develop entirely new enzymes for the production of diverse aromatic polyketides. Moreover, software such as PKMiner [27][33] or antiSMASH [28][34] can be employed to discover type II PKSs suitable for the biosynthesis of desired or novel aromatic polyketides. Such recent advancements in the heterologous expression of type II PKSs will contribute to the industrial production of medicinal polyketides as well as novel non-natural polyketide derivatives.
