Liquid crystals are generally referred to as substances that blend the structure and properties of solid and liquid states; they share with liquids the ability to flow but also exhibit some structural arrangement similarities with solids. With so many compounds synthesized, liquid crystals containing metals, also known as metallomesogens, have become a major subject of study. The incorporation of metal into organic matrices enhances and induces unique magnetic, spectroscopic, and redox properties of the resulting materials. At least one liquid crystalline complex has been reported in the literature for most metals.
- copper(I)
- metallomesogens
- luminescence
- complex
- ligand
1. Introduction
Copper(I) complexes have not been studied to a great extent as luminescent materials in the liquid crystalline state, but they do possess luminescence properties with great potentials [13,14,15,16,17,18,19,20,21,22][1][2][3][4][5][6][7][8][9][10]. Copper, which is somewhat abundant and affordable, is a suitable alternative to noble metal complexes [23,24][11][12]. The ratio of triplet to singlet excitons is 3:1; consequently, for luminescent materials to be used in OLEDs, they should essentially be able to harvest all the excitons. Because copper(I) complexes exhibit various metal-to-ligand charge-transfer (MLCT) behaviors, they can induce spin orbital coupling of the triplet and singlet states, leading to small energy separations between the energy levels [25][13]. This allows for reverse intersystem crossing (RISC), i.e., singlet harvesting, resulting in thermally activated delayed fluorescence (TADF) [26][14]. Therefore, liquid crystals based on copper(I) complexes have considerable promise for producing effective luminescent materials for a wide range of optical or electro-optical applications due to the large diversity of possible structures, including mononuclear or polynuclear complexes, and the great potential of emission behavior. In addition, the range of coordination geometries (such as linear, plan-trigonal, or tetrahedral) combined with the ligands’ structural design provide a significant benefit for controlling the LC properties, including their enhanced thermal stability and mesophase type related to both calamitic and discotic materials.
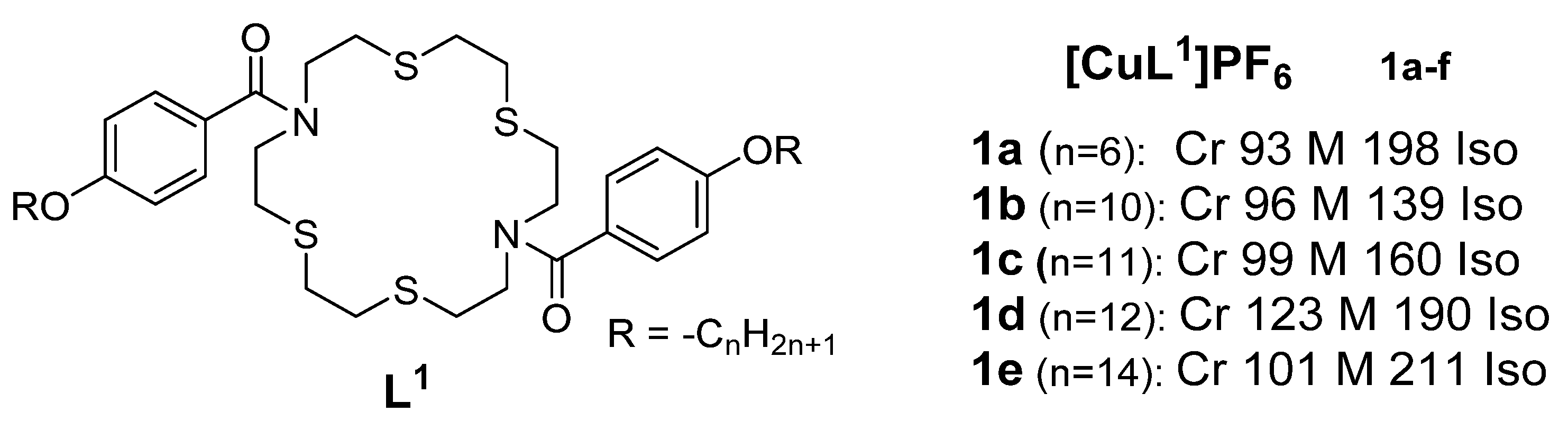
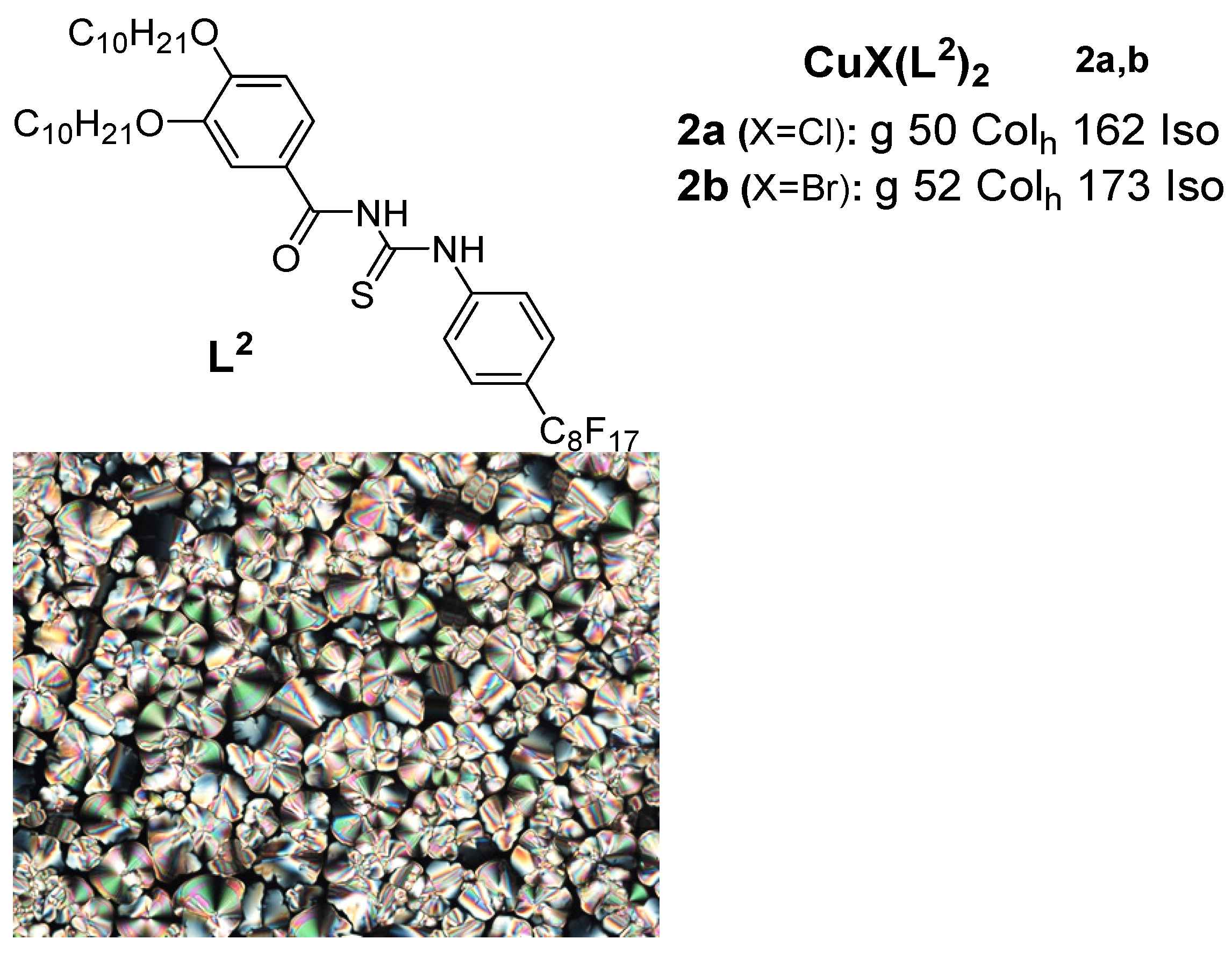
2. Copper(I) Metallomesogens with Sulfur-Containing Ligands
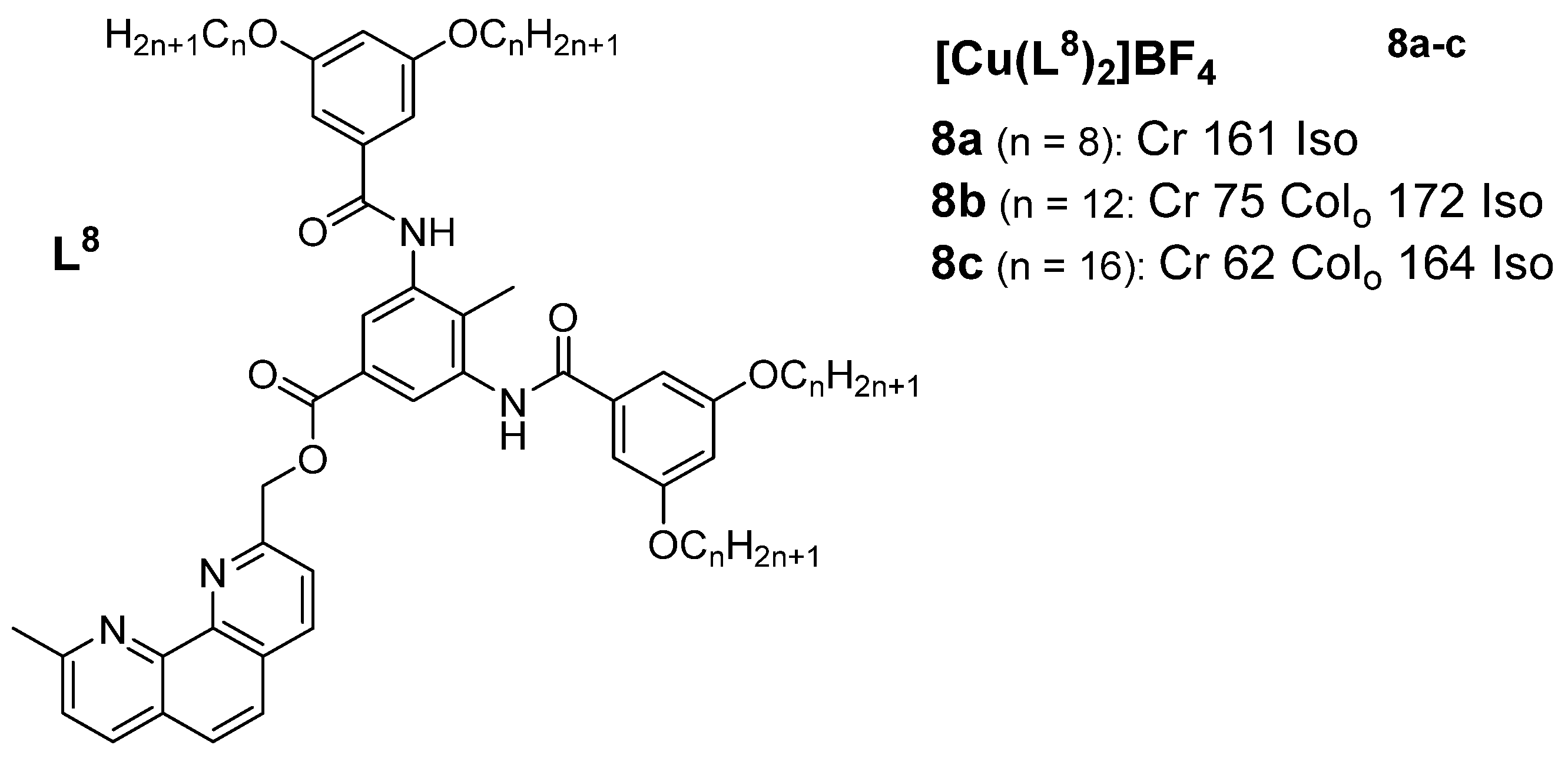
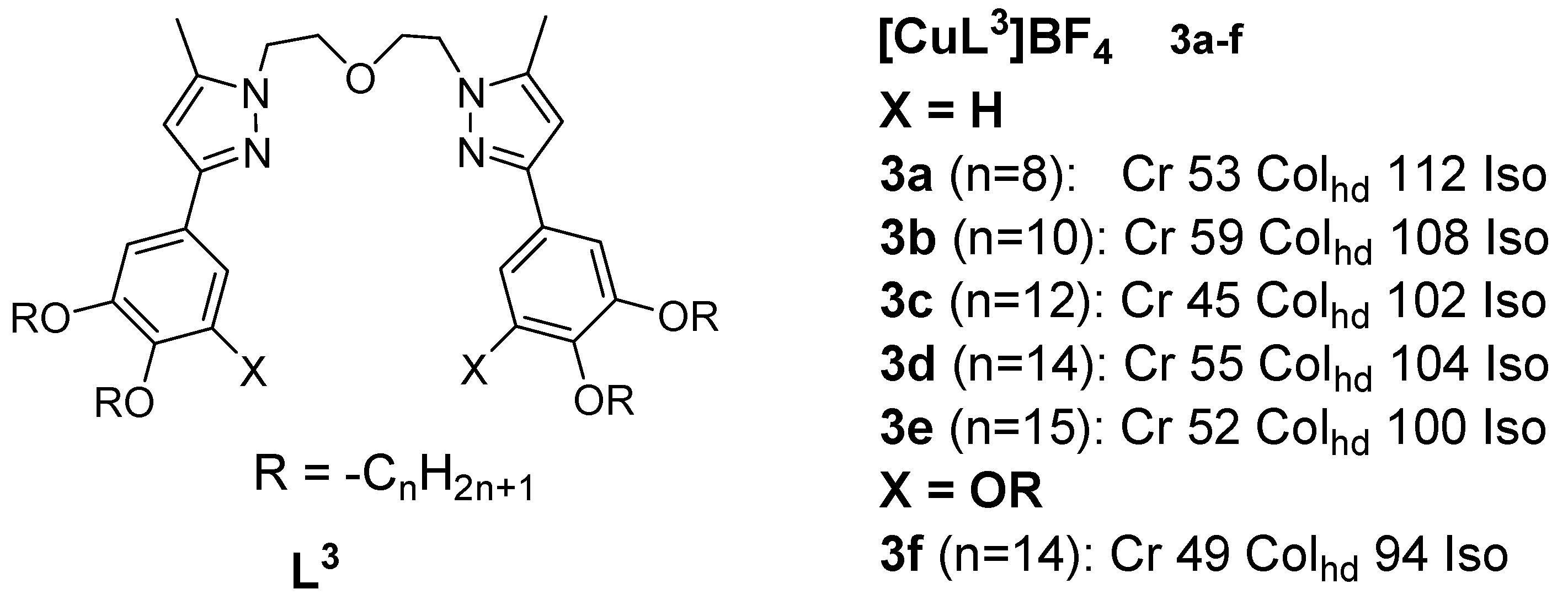
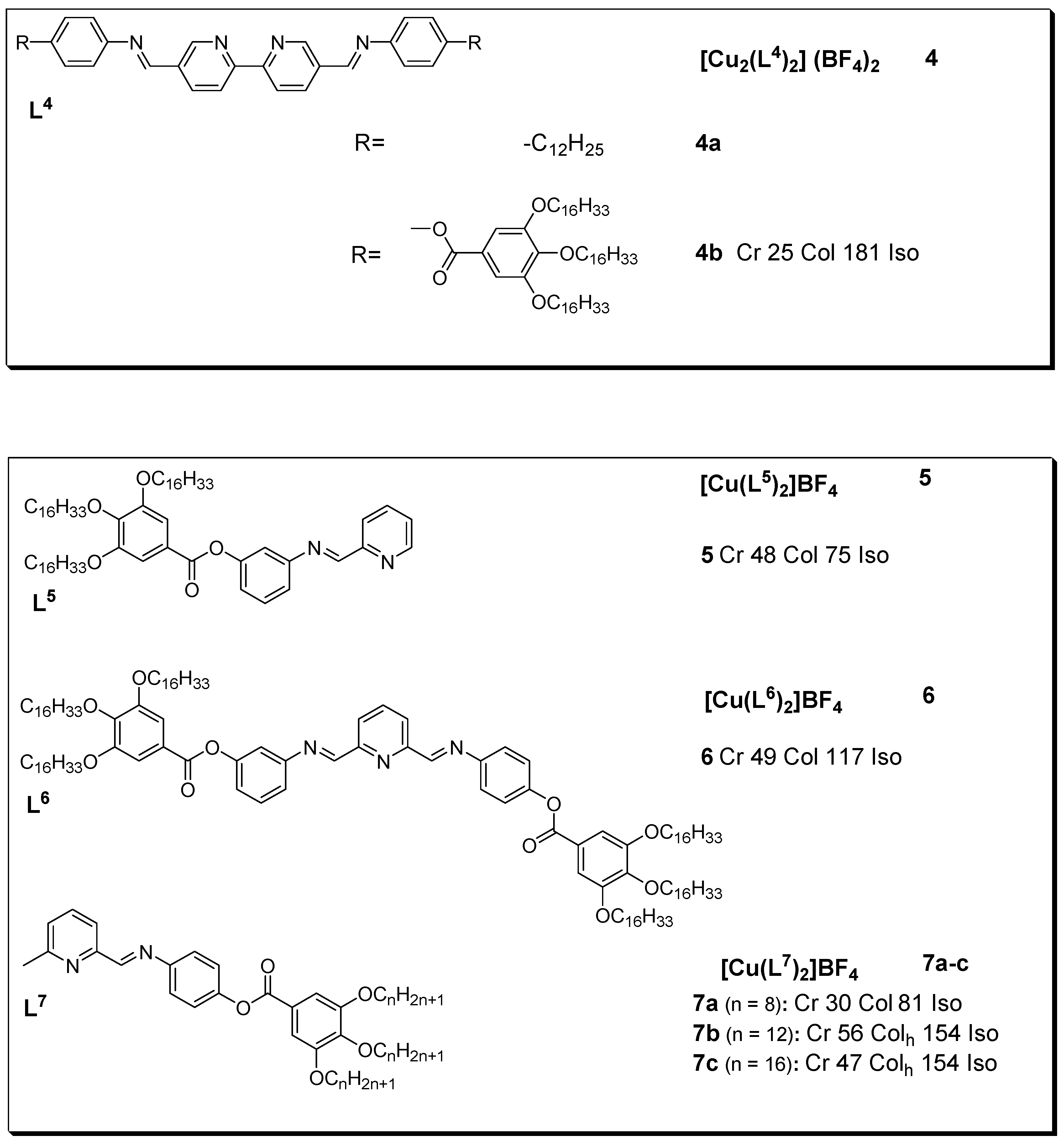
3. Copper(I) Metallomesogens with N-Donor Ligands
References
- Tudor, C.A.; Iliş, M.; Secu, M.; Ferbinteanu, M.; Cîrcu, V. Luminescent heteroleptic copper(I) complexes with phosphine and N-benzoyl thiourea ligands: Synthesis, structure and emission properties. Polyhedron 2022, 211, 115542.
- Favarin, L.R.V.; Rosa, P.P.; Pizzuti, L.; Machulek, A.; Caires, A.R.L.; Bezerra, L.S.; Pinto, L.M.C.; Maia, G.; Gatto, C.C.; Back, D.F.; et al. Synthesis and structural characterization of new heteroleptic copper(I) complexes based on mixed phosphine/thiocarbamoyl-pyrazoline ligands. Polyhedron 2017, 121, 185–190.
- Chan, K.C.; Cheng, S.C.; Lo, L.T.L.; Yiu, S.M.; Ko, C.C. Luminescent Charge-Neutral Copper(I) Phenanthroline Complexes with Isocyanoborate Ligand. Eur. J. Inorg. Chem. 2018, 2018, 897–903.
- Bergmann, L.; Friedrichs, J.; Mydlak, M.; Baumann, T.; Nieger, M.; Bräse, S. Outstanding luminescence from neutral copper(i) complexes with pyridyl-tetrazolate and phosphine ligands. Chem. Commun. 2013, 49, 6501–6503.
- Enikeeva, K.R.; Shamsieva, A.V.; Strelnik, A.G.; Fayzullin, R.R.; Zakharychev, D.V.; Kolesnikov, I.E.; Dayanova, I.R.; Gerasimova, T.P.; Strelnik, I.D.; Musina, E.I.; et al. Green Emissive Copper(I) Coordination Polymer Supported by the Diethylpyridylphosphine Ligand as a Luminescent Sensor for Overheating Processes. Molecules 2023, 28, 706.
- Tsuge, K.; Chishina, Y.; Hashiguchi, H.; Sasaki, Y.; Kato, M.; Ishizaka, S.; Kitamura, N. Luminescent copper(I) complexes with halogenido-bridged dimeric core. Coord. Chem. Rev. 2016, 306, 636–651.
- Sun, Y.; Lemaur, V.; Beltrán, J.I.; Cornil, J.; Huang, J.; Zhu, J.; Wang, Y.; Fröhlich, R.; Wang, H.; Jiang, L.; et al. Neutral Mononuclear Copper(I) Complexes: Synthesis, Crystal Structures, and Photophysical Properties. Inorg. Chem. 2016, 55, 5845–5852.
- Borges, A.P.; Carneiro, Z.A.; Prado, F.S.; Souza, J.R.; Silva, L.H.F.E.; Oliveira, C.G.; Deflon, V.M.; de Albuquerque, S.; Leite, N.B.; Machado, A.E.H.; et al. Cu(I) complexes with thiosemicarbazides derived from p-toluenesulfohydrazide: Structural, luminescence and biological studies. Polyhedron 2018, 155, 170–179.
- Brown, C.M.; Li, C.; Carta, V.; Li, W.; Xu, Z.; Stroppa, P.H.F.; Samuel, I.D.W.; Zysman-Colman, E.; Wolf, M.O. Influence of Sulfur Oxidation State and Substituents on Sulfur-Bridged Luminescent Copper(I) Complexes Showing Thermally Activated Delayed Fluorescence. Inorg. Chem. 2019, 58, 7156–7168.
- Bergmann, L.; Braun, C.; Nieger, M.; Bräse, S. The coordination- and photochemistry of copper(i) complexes: Variation of N^N ligands from imidazole to tetrazole. Dalton Trans. 2018, 47, 608–621.
- Li, X.; Xie, Y.; Li, Z. Diversity of Luminescent Metal Complexes in OLEDs: Beyond Traditional Precious Metals. Chem. Asian J. 2021, 16, 2817–2829.
- Dumur, F. Recent advances in organic light-emitting devices comprising copper complexes: A realistic approach for low-cost and highly emissive devices? Org. Electron. 2015, 21, 27–39.
- Yersin, H.; Czerwieniec, R.; Shafikov, M.Z.; Suleymanova, A.F. TADF Material Design: Photophysical Background and Case Studies Focusing on Cu(I) and Ag(I) Complexes in Highly Efficient OLEDs: Materials Based on Thermally Activated Delayed Fluorescence; Yersin, H., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2018; pp. 1–60.
- Housecroft, C.E.; Constable, E.C. TADF: Enabling luminescent copper(i) coordination compounds for light-emitting electrochemical cells. J. Mater. Chem. C 2022, 10, 4456–4482.
- Neve, F.; Ghedini, M.; Levelut, A.-M.; Francescangeli, O. Ionic metallomesogens. Lamellar mesophases in copper(I) azamacrocyclic complexes. Chem. Mater. 1994, 6, 70–76.
- Espinet, P.; Carmen Lequerica, M.; Martín-Alvarez, J.M.Â. Synthesis, Structural Characterization and Mesogenic Behavior of Copper(I) n-Alkylthiolates. Chem. Eur. J. 1999, 5, 1982–1986.
- Dance, I.G.; Fisher, K.J.; Banda, R.M.H.; Scudder, M.L. Layered Structure of Crystalline Compounds AgSR. Inorg. Chem. 1991, 30, 183–187.
- Iliş, M.; Cîrcu, V. Discotic Liquid Crystals Based on Cu(I) Complexes with Benzoylthiourea Derivatives Containing a Perfluoroalkyl Chain. J. Chem. 2018, 2018, 7943763.
- Lin, H.-D.; Lai, C.K. Ionic columnar metallomesogens formed by three-coordinated copper(I) complexes. J. Chem. Soc. Dalton Trans. 2001, 2383–2387.
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases—Structure, Importance and Classification. Molecules 2022, 27, 787.
- Khalaji, A.D. Structural Diversity on Copper(I) Schiff Base Complexes, Current Trends in X-Ray Crystallography. Chandrasekaran, Q., Ed.; InTech: London, UK, 2011; pp. 161–190. ISBN 978-953-307-754-3.
- Jamain, Z.; Azman, A.N.A.; Razali, N.A.; Makmud, M.Z.H. A Review on Mesophase and Physical Properties of Cyclotriphosphazene Derivatives with Schiff Base Linkage. Crystals 2022, 12, 1174.
- Rananavarem, S.B.; Pisipati, V.G.K.M. An Overview of Liquid Crystals Based on Schiff Base Compounds, Liquid Crystalline Organic Compounds and Polymers as Materials XXI Century: From Synthesis to Applications; Iwan, A., Schab-Balcerzak, E., Eds.; Transworld Research Network: Trivandrum, Kerala, 2011; ISBN 978-81-7895-496-7.
- Hoshino, N. Liquid crystal properties of metal–salicylaldimine complexes.: Chemical modifications towards lower symmetry. Coord. Chem. Rev. 1998, 174, 77–108.
- Torroba, J.; Bruce, D.W. Comprehensive Inorganic Chemistry II: From Elements to Applications, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 8, pp. 837–917.
- El-Ghayoury, A.; Douce, L.; Skoulios, A.; Ziessel, R. Cation-Induced Macroscopic Ordering of Non-Mesomorphic Modules—A New Application for Metallohelicates. Angew. Chem. Int. Ed. 1998, 37, 2205–2208.
- Douce, L.; Diep, T.H.; Ziessel, R.; Skoulios, A.; Césario, M. Columnar liquid crystals from wedge-shaped tetrahedral copper(i) complexes. J. Mater. Chem. 2003, 13, 1533–1539.
- Douce, L.; El-Ghayoury, A.; Ziessel, R.; Skoulios, A. Columnar mesophases from tetrahedral copper(I) cores and Schiff-base derived polycatenar ligands. Chem. Commun. 1999, 2033–2034.
- Ziessel, R.; Pickaert, G.; Camerel, F.; Donnio, B.; Guillon, D.; Cesario, M.; Prangé, T. Tuning Organogels and Mesophases Will Phenanthroline Ligands and Their Copper Complexes by Inter- to Intramolecular Hydrogen Bonds. J. Am. Chem. Soc. 2004, 126, 12403–12413.