Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Fanny Huang and Version 1 by Laura Kate Gadanec.
Taurine, black pepper, and the major terpene constituents found in black pepper (i.e., β-caryophyllene; α-pinene; β-pinene; α-humulene; limonene; and sabinene) that are present in PhytoCann BP® have been shown to have cardioprotective effects based on anti-inflammatory, antioxidative, anti-hypertensive and anti-atherosclerotic mechanisms.
- black pepper
- taurine
- terpenes
1. Introduction
Natural products are well-known for their therapeutic value [1]. Throughout human history, they have played an important role in traditional medicine by treating a wide range of pathological ailments, offering effective alternatives to modern medicine [2]. Thanks to recent advances in scientific research and growing environmental awareness [3], there has been a significant increase in the use of natural and plant-based products to promote synergistic biological health benefits [4,5,6][4][5][6]. This is particularly important in western society where a shift in the modern dietary regimen towards a “Western diet” and an increase in sedentary lifestyles is believed to be, in part, responsible for the substantial increase in the prevalence of cardiovascular complications [7,8][7][8].
Cardiovascular diseases (CVDs) refer to a broad range of conditions affecting the heart and blood vessels [9]. They register a global annual death toll of more than 19.1 million and are the most prevalent cause of morbidity and mortality [10]. Alarmingly, the World Health Organization estimates that CVDs will contribute to over 22 million deaths in 2030 [11]. CVDs encompass a variety of diseases, including hypertension, atherosclerosis, coronary artery disease (CAD), heart failure (HF), myocardial infarction (MI) and stroke [9]. Atherosclerosis and hypertension are the leading causes of CVDs and are the most common underlying diatheses for most deaths [12]. Independent risk factors for CVDs include hypertension, hyperhomocysteinemia (HHcy), dyslipidemia, type-2 diabetes, and cholesterol [12,13,14][12][13][14]. The pathology of CVDs is complex, and oxidative stress, hyper-inflammation, endothelial dysfunction, and hyperlipidemia have been reported as common phenotypes in the pathogenesis of CVDs and have become the main targets of therapeutic intervention [15,16][15][16]. Conventional therapy commonly used to treat CVDs can be costly and may produce adverse side effects, unwanted drug–drug interactions and life-threatening toxicities [17], and thus, alternative approaches have been considered, including physical activity, as well as the use medicinal natural product supplementation, for CVD treatment [18,19,20][18][19][20].
Taurine, a natural product, is a non-toxic, semi-essential sulfur-containing amino acid (Figure 1A) that is abundant in human cardiac and vascular tissues [21,22][21][22]. Although synthesized endogenously from methionine and cysteine [23], the majority of taurine is obtained through dietary intake (e.g., seafood, meat, poultry and eggs) [21]. It is well-established that taurine has diverse physiological functions in humans and is seen to protect against several pathophysiological conditions [22,24,25][22][24][25]. Most of the effects mediated by taurine are reported in the cardiovascular system and include modulating ion transporters, membrane stabilization, anti-oxidation, anti-hyperlipidemia, anti-platelet, and anti-inflammatory activity, as well as modulation of blood pressure and vascular tone [22,24,25][22][24][25].
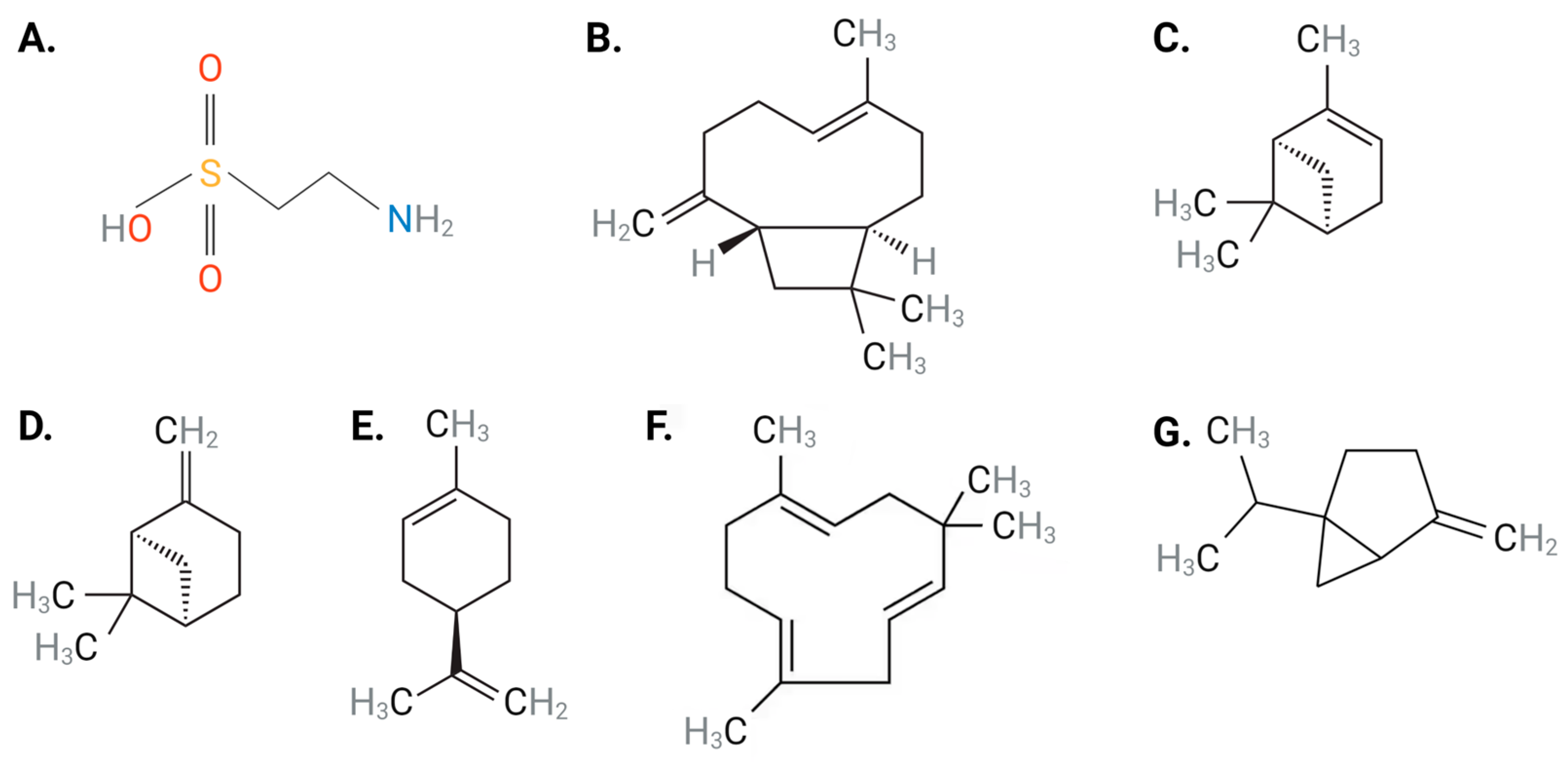
Figure 1. Chemical structures of (A) taurine, (B) BCP, (C) α-pinene, (D) β-pinene, (E) limonene, (F) α-humulene and (G) sabinene. Abbreviations: BCP, β-caryophyllene. Black: carbon, gray: hydrogen: blue: nitrogen; red: oxygen, yellow: sulfur Figure designed with Biorender.com.
Piper nigrum, commonly known as black pepper, has been used to treat a variety of conditions [26]. The therapeutic value of black pepper is attributed to its constituents, terpenes or terpenoids, which are a diverse class of organic compounds present in essential oils obtained from plants [26,27][26][27]. Terpenes consist of over 30,000 compounds whose functional groups include alcohols, aldehydes, or ketones [28]. Abundant terpenes found in black pepper consist of β-caryophyllene (BCP) (Figure 1B), α-pinene (Figure 1C), β-pinene (Figure 1D), limonene (Figure 1E), α-humulene (Figure 1F), and sabinene (Figure 1G) [29]. The therapeutic merit of black pepper has been attributed to the ability of BCP to specifically activate cannabinoid 2 receptor (CB2R), resulting in downstream signaling pathways that mediate anti-inflammatory, anti-atherosclerotic and anti-oxidative properties, which have been correlated with beneficial cardiovascular effects [30]. Unlike BCP, studies involving α-pinene [31[31][32],32], β-pinene [31,32][31][32] and α-humulene [33] have demonstrated the inability of these terpenes to interact with CB2R. Several studies have noted the significant cardiovascular-protective effects of black pepper and its terpenes against a range of CVDs, including hypertension, atherosclerosis, CAD, and HF due to their anti-inflammatory, anti-oxidative, anti-hyperlipidemia and blood pressure-lowering abilities [34,35][34][35]. As such, black pepper provides novel therapeutic strategies to target CVD initiation and progression.
2. Anti-Inflammatory Effects of Taurine and Terpenes
Acute and chronic inflammation play key roles in the pathogenesis, progression, and severity of CVDs [36]. Hypertension, atherosclerosis and CAD are characterized as chronic inflammatory diseases and are clinically associated with increased expression of important pro-inflammatory receptors (e.g., toll-like receptors (TLR) and receptor for advanced glycation end-products (RAGE)) and molecules (e.g., high-mobility group box-1 (HMGB-1), interleukin (IL)-1β, IL-6, tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), nitric oxide (NO), prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS), and cyclooxygenase (COX)-2) [8,37][8][37]. Thus, their downregulation and inhibition have been reported in literature as potential therapeutic targets against CVDs [38]. Taurine and terpenes contain general anti-inflammatory effects (Table 1), which may be useful in combination therapy as due to their abilities to suppress the expression of pro-inflammatory cytokines and alleviate cell and tissue damage in multiple disease states [39,40,41,42][39][40][41][42]. The two natural compounds significantly mitigate the expression of pro-inflammatory mediators (e.g., IL-1α, IL-1β, IL-6, IL-13, IL-17, TNF-α, interferon-γ, MCP-1, and vascular endothelial growth factor) in order to reverse the severity of inflammation-mediated injury [42,43][42][43]. Administration of taurine (100 mM) in Wistar rats 30 days after coronary artery occlusion-induced ischemia significantly decreased myocardial infarct size and reduced expression of IL-6 and TNF-α, suggesting that taurine provides anti-inflammatory cardio-protective effects [44]. Understanding the anti-inflammatory targets of taurine and terpenes may provide further evidence of their therapeutic use for CVDs (Figure 2, Table 1), as well as other conditions such as inflammatory bowel disease [45], rheumatoid arthritis [45], and traumatic brain injury where inflammation is a well-known critical event [43].
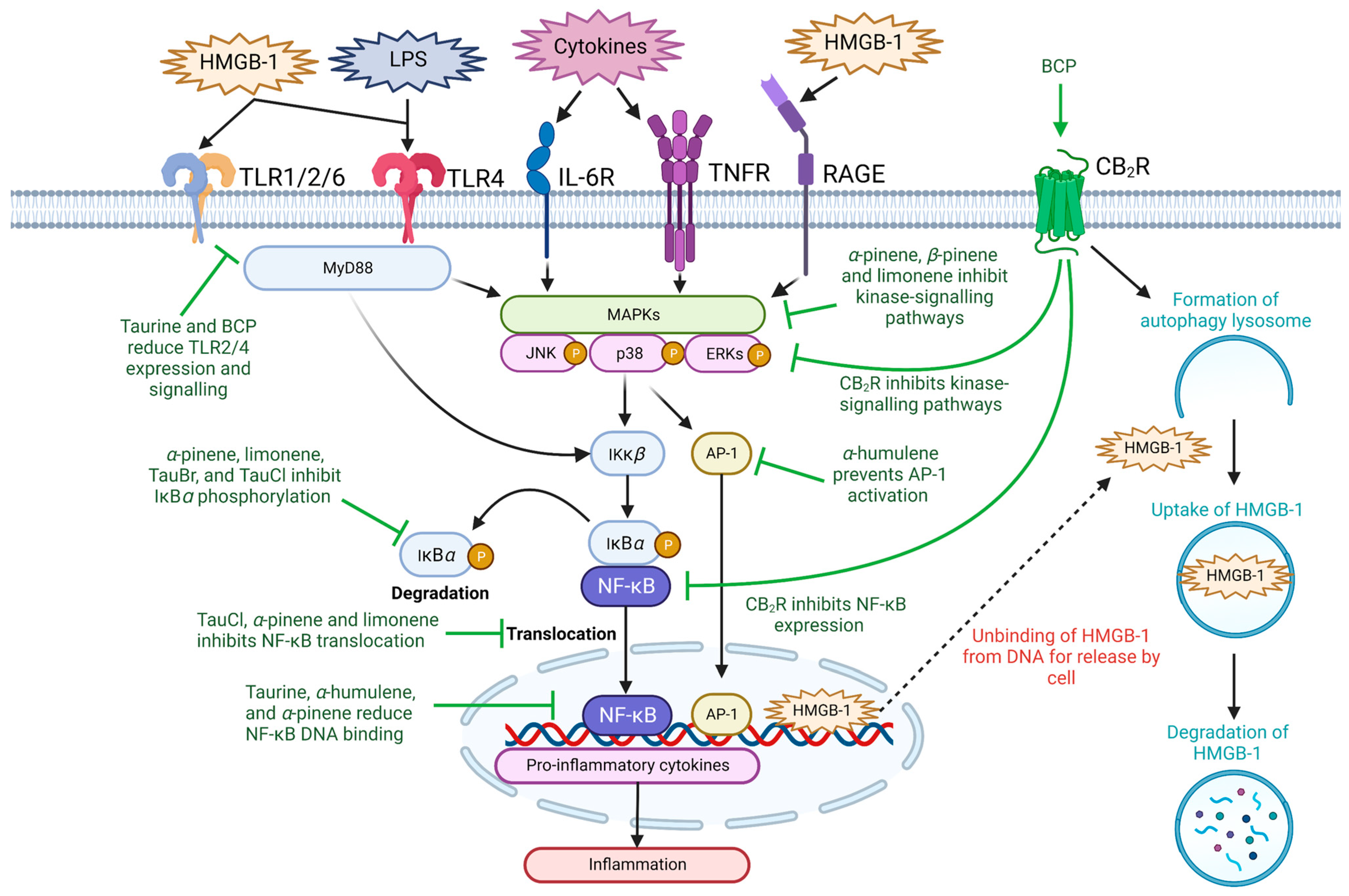
Figure 2. The anti-inflammatory abilities of taurine and terpenes through modulating NF-κB signaling. Taurine, its metabolites, and the major terpenes present in black pepper may suppress inflammation through preventing NF-κB-mediated proinflammatory cytokine production. The current literature suggests that this occurs through inhibition of upstream kinase signaling pathways, such as receptors of the immune system (i.e., TLR, RAGE, TNFR and IL-6R), MAPK/p38/ERK/JAK-signaling, release of HMGB-1, NF-κB activation, translocation or reducing NF-κB DNA binding ability. Ɑ-humulene may also inhibit proinflammatory cytokine production through downregulation of AP-1 signaling. Abbreviations: AP-1, activator protein-1; BCP, β-caryophyllene; CB2R, cannabinoid type 2 receptor; ERK, extracellular signal-regulated kinase; HMGB-1, high-mobility group box-1; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha; IκKβ, nuclear factor kappa-B kinase subunit beta; IL-6R, interleukin 6 receptor; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MyD88, myeloid differentiation protein 88; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; P, phosphorylation; RAGE, receptor for advanced glycation end products; TLR, toll-like receptor; TNFR, tumor necrosis factor receptor. Figure designed with Biorender.com.
2.1. Inflammatory Signaling Pathways
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a central transcription regulator that plays a critical role in the activation of inflammatory responses by inducing transcription of pro-inflammatory genes [46]. During basal conditions, NF-κB is bound to the inhibitory protein nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IκBα) within the cytosol [47]. Upon stimulation, the inhibitor of NF-κB subunit beta (IκKβ) phosphorylates and degrades IκBα, allowing NF-κB dimers p50 and p65 to translocate towards the nucleus where they upregulate the activation of pro-inflammatory genes [48]. The phosphorylation of IκBα is triggered by a diverse range of stimuli, including various ligands for IL-6 receptors, TNF receptor (TNFR), and pattern-recognition receptors (PRRs) (e.g., TLR and RAGE) [49[49][50][51],50,51], which can activate kinase-signaling transduction pathways consisting of p38-mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK) and Janus kinase (JAK) [52].
Taurine and terpenes both exert anti-inflammatory effects through inhibition of NF-κB and kinase-signaling pathways [53,54,55][53][54][55]. An increase in taurine metabolites, chloro-taurine (TauCl) and taurine-bromamine (TauBr) have been shown to modulate the myeloid-differentiation factor-88 (MyD88)-dependent TLR/NF-κB inflammatory signaling pathway in both human and mouse macrophages to disrupt the phosphorylation and degradation of IκBα [56[56][57],57], as well as prevent the activation and subsequent translocation of NF-κB [56,58][56][58]. Additionally, taurine can significantly reduce the expression signal transducer and activator of transcription 3 (STAT) 3 in the JAK-STAT signaling pathway [44], which is shown to play a vital role in cardio-protection, as inflammation associated with myocardial ischemia is mitigated through inhibition of JAK-STAT3-signaling pathways [44]. Black pepper terpenes have also been shown to attenuate pro-inflammatory cytokine expression through downregulation of kinase signaling pathways and inhibition of IκBα phosphorylation [44,59][44][59]. Limonene blocks the phosphorylation of IκBα and NF-κB to suppress lipopolysaccharide (LPS)-induced inflammation in acute lung injury [59]. α-pinene has been shown to inhibit IκKβ activation and prevent NF-κB translocation [60]. In human chondrocytes, α-pinene (200 µg/mL) suppressed IL-1β-induced inflammation through suppression of NO, iNOS, matrix metallopeptidase (MMP)-1, and IL-13 pro-inflammatory mediators [61]. This anti-inflammatory effect was produced by the inhibition of c-Jun N-terminal kinase (JNK) phosphorylation. Additionally, α-pinene has also been shown to attenuate MAPK and NF-κB activation, resulting in the decreased expression of pro-inflammatory factors IL-6, TNF-α, NO, iNOS, and COX-2 [44]. The terpenes BCP, α-humulene, and α-pinene have been shown to inhibit the expression of NO synthase and COX-2 through inhibition of MAPK action through IKK-B inhibition [38], while limonene was shown to mitigate pro-inflammatory cytokines secretion as well as rescue iNOS, MMP-1, and MMP-3 gene transcription through inhibition of MAPK/JNK-dependent NF-κB activation pathways [62]. Pre-treatment with limonene (25, 50 and 75 mg/kg) inhibited release of pro-inflammatory cytokines (i.e., TNF-α, IL-1β and IL-6) and prevented phosphorylation of p38-MAPK, IκBα, NF-κB p65, JNK and ERK in mice with lipopolysaccharide (LPS)-induced acute lung injury [59]. Likewise, both α-pinene and β-pinene reduce the expression of genes associated with LPS-induced inflammation [63], suggesting that terpenes may mediate anti-inflammatory effects through regulation of kinase and NF-κB gene signaling.
BCP has been identified as a CB2R agonist [64], which is primarily expressed in immune tissues and is known to modulate immune cell function to provide anti-inflammatory effects [65]. Exogenous administration of CB2R agonists is observed to inhibit inflammation by reducing the production of pro-inflammatory cytokines and attenuating oxidative stress in various inflammatory diseases [66,67][66][67]. BCP has been observed to inhibit the activation of NF-κB, thus attenuating the production of inflammatory mediators. Furthermore, this effect disappears with CB2R−/− knockout expression, suggesting that BCP exerts its anti-inflammatory responses through activation of CB2R [68]. The cardioprotective effects of BCP on isoproterenol (ISO)-induced myocardial injury in Wister rats through activation of the CB2R has been observed. BCP modulated MAPK/ERK/JNK/NF-κB signaling to significantly suppress the expression of IL-1β, IL-6, TNF-α, iNOS, and COX-2 [69]. Additionally, this comprehensive study reported that BCP also attenuated mitochondrial dysfunction and improved the atherogenic index via a CB2R-independent reduction in dyslipidemia [69]. BCP has been shown to inhibit the expression of vascular cell adhesion molecule-1 [70], which is known to promote the adhesion of macrophages to the vascular endothelium in the development of atherogenic plaque [71]. These studies suggest that both taurine and terpene extracts present in black pepper may combine to exert effective anti-inflammatory responses and thereby provide beneficial cardioprotective effects in CVDs.
The nucleotide-binding domain, leucine-rich containing family, pyrin domain-containing 3 (NLRP3) inflammasome is a critical component of the innate immune system and is responsible for the production of pro-inflammatory cytokines in response to cellular damage [72]. Recently, NLRP3 has been shown to play an indispensable role in the development of vascular diseases, including hypertension, atherosclerosis, and CAD, as well as diabetes and neurological disorders [73]. Increasing evidence supports NLRP3 as a new target to inhibit inflammatory diseases [74]. NLRP3 is regulated by NF-κB signaling pathways; therefore, taurine and terpenes have the ability to inhibit NF-κB as researchers have previously highlighted a pathway for preventing NLRP3 activation. Although taurine has been shown to attenuate in vitro and ex vivo inflammation through inhibition of NF-κB-dependent NLRP3 activation [75[75][76],76], studies on the isolated effect of black pepper terpenes on inflammasome inhibition are limited. A study conducted on the essential oil of pomelo peel, in which limonene (55.92%) was the most abundant compound, found that it significantly inhibited inflammation mediated by NLRP3 activation in rats [77]. Additionally, Makauy leaf ethanol extract consisting of limonene (5.6%) and BCP (4.9%) inhibited the in vitro expression of NLRP3 in J774A.1 mouse macrophage cells stimulated with LPS-induced inflammation [78]. Lastly, CB2R agonist JWH-133 has been shown to produce cardioprotective effects against MI in rats by suppressing NLPR3-related mechanisms, suggesting that BCP, a CB2R agonist, may also suppress inflammation through NLPR2 inhibition [79].
Collectively, these studies highlight the effects that taurine and black pepper terpenes have on inhibiting NF-κB, kinase- and inflammasome-signaling pathways and suppressing the expression of pro-inflammatory mediators. The potential combined use of taurine and terpenes may have synergistic effects in exerting effective anti-inflammatory responses, potentially providing effective treatment of CVDs.
2.2. High-Mobility Group Box-1
HMGB-1 is an evolutionarily preserved, architectural, non-histone chromosome binding peptide that is found abundantly within the nucleus bound to DNA [80,81][80][81]. HMGB-1 facilities nuclear homeostasis by coordinating important nuclear functions, such as maintaining stability of the genome, acting as a chaperone for DNA, and regulating DNA transcription, replication, recombination, and repair [80,81][80][81]. Outside the nucleus, HMGB-1 drives cell stress responses by acting as a dynamic alarmin signal (which can also be referred to as a danger associated molecular pattern (DAMP)) that engages with PRRs of the immune system (e.g., TLR1/2/6, TLR4 and RAGE) to promote inflammation through the canonical NF-κB signaling pathway (discussed above) [81,82,83,84][81][82][83][84]. Elevated levels of HMGB-1 have been demonstrated in animal models of atherosclerosis, hypertension, CAD, MI, and HF [84]. Clinical studies have also reported increased HMGB-1 serum levels in patients with CAD [85,86][85][86], peripheral artery [87] and HHcy [88], suggesting that HMGB-1 has a critical pathogenic role during development and progression of CVDs. While the correlation between taurine and HMGB-1 has yet to be investigated in the literature, a study involving patients with bladder cancer noted elevated levels of taurine upregulate gene 1 (TUG1; long non-coding RNA transcripts that require taurine for gene upregulation) and the reversal of TUG1 knockdown by overexpression of HMGB-1 [89], thus suggesting a possible unknown mechanism between taurine, TUG1 and HMGB-1. The role that taurine and BCP have in attenuating HMGB-1-mediated inflammation and dysfunction remains elusive; however, there is evidence to suggest that there may be an unknown interlinking mechanism between taurine and HMGB-1 [90]. In mice with HMGB-1 deletion in intestinal epithelial cells, a diet high in fat, cholesterol and fructose increased taurine concentration in serum collected from portal blood samples when compared with wild type controls [90].
Moreover, BCP and CB2R activation may lower HMGB-1, contributing to a reduction in inflammation. CB2R knockout mice challenged with LPS to induce sepsis displayed reduced survival rate and significantly elevated serum IL-6, TNF-α and HMGB-1 levels when compared with CB2R expressing littermates [91]. Furthermore, treatment with GW405833 (10 mg/kg, a CB2R agonist) was able to significantly reduce serum levels of TNF-α, IL-6, and HMGB-1 6 h after LPS injection (5 mg/kg) [91]. Furthermore, activation of CB2R with GW405833 has been shown to induce intracellular HMGB-1 degradation via the autophagy-lysosome pathway in macrophages without modulating HMGB-1 mRNA expression [92], thus potentially preventing its release into the extracellular milieu where it can engage with immune system receptors and activate pro-inflammatory downstream signalling pathways. BCP’s anti-inflammatory properties may also be due to its ability to reduce HMGB-1 and its endogenous receptors as BCP was able to attenuate increased serum levels of HMGB-1 and suppressed protein expression of TLR4 and RAGE and their downstream inflammatory molecules (i.e., ERK, p38 and JNK phosphorylation, NF-κB, early growth response protein-1, and macrophage inflammatory protein-2 protein) in Kupffer cells from mice with galactosamine and LPS-induced hepatic injury [93].
Although the relationship between taurine, terpenes and HMGB1 remains elusive, these findings suggest that targeting HMGB-1-mediated inflammation through a combination of taurine and black pepper terpenes may be a potential mechanism to reduce inflammation associated with CVDs.
2.3. Toll-like Receptors
TLRs are integral to immunity as they provide host surveillance by detecting molecular signatures present on pathogens (referred to as pathogen-associated molecular patterns) and launch mechanisms of inflammation to neutralize and eliminate infection [94]. Importantly, TLRs also provide protection against tissue injury in the absence of pathogenic infiltration by identifying DAMPs (e.g., HMGB-1) secreted by damaged, injured, and dying cells [94,95][94][95]. However, unregulated TLR activation results in a vicious cycle of unresolving inflammation and has been demonstrated to be fundamental in the development of CVDs. Thus, they have become a target to attenuate disease development [95,96][95][96]. Specifically, taurine has been shown to suppress inflammation via modulation of the TLR2 and TLR4 [97,98,99][97][98][99] signaling pathways. TLR2 and 4 are able to recognize bacterial cell wall components (e.g., lipopeptides, peptidoglycan and LPS), and stimulation of TLR4 causes receptor homodimerization of the MyD88- and Toll/IL-1 receptor domain-containing adaptor-inducing interferon-β (TRIF)-dependent signaling cascade, whereas TLR2 activation requires recruitment of TLR1 or 6 for normal receptor function via the MyD88-dependent pathway [100,101][100][101]. Male Sprague-Dawley rats challenged with LPS (25 mg/kg; TLR4 activating ligand) to induce acute lung injury showed that those which were pre-treated with taurine supplemented drinking water had decreased infiltration and activation of neutrophils and macrophages. Further, they lowered levels of TLR4, MyD88, NF-κB and p65, reduced pro-inflammatory cytokines (IL-6, IL-18) and increased anti-inflammatory cytokines (IL-4, IL-10) in serum and lung tissues of the treated rats [97]. Similarly, taurine provides hepatic protection by targeting the TLR4/MyD88/NF-κB pathway in rat models of chronic alcohol-fed [98] and thioacetamide-induced [99] liver injury, resulting in decreased lymphocyte infiltration and reduced inflammation (TLR4, MyD88, IκB, NF-κB, iNOS, IL-6, IL-1β, and TNF-α). Regarding TLR2, taurine has been shown to reduce neutrophil activation of MAPK signaling following Streptococcus uberis (TLR2 activating ligand) infection [102]. A supporting study also showed that pre-treatment of taurine in rat models with Streptococcus uberis mastitis had significantly decreased expression of iNOS and TNF-α, which was associated with the downregulation of TLR2 and NF-κB mRNA expression, as well as inhibition of the DNA binding activity of NF-κB [103].
Like taurine, terpenes may also exert anti-inflammatory effects by modulation of the TLR signaling cascade. In LPS activated RBL-2H3 cells (derived from a basophilic leukemia cell line), α-pinene, β-pinene and limonene displayed anti-inflammatory abilities by reducing the expression of genes associated with LPS-mediated inflammation [63]. Likewise, α-pinene attenuated activation of the MAPK and NF-κB pathways and reduced production of IL-6, TNF-α, NO, iNOS and COX-2 following LPS stimulated mouse intraperitoneal macrophages [104]. However, in human chondrocytes, α-pinene elicited a more potent inhibition of IL-1β-induced inflammation, NF-κB and JNK activation and expression of iNOS compared with β-pinene and limonene [61]. Importantly, BCP has been shown to ameliorate ISO-induced (85 mg/kg) MI through modulation of the heat shock protein-60/TLR2/4/MyD88/NF-κB pathway. This study reported that BCP treatment was able to markedly reduce infarct size, normalized electrocardiogram and blood pressure parameters and significantly reduced protein expression of MyD88, TLR2, TLR4, and TIR-domain-containing adaptor-inducing interferon-β (accessory molecule of TLR signal transduction) and levels of inflammatory markers (i.e., IL-1β and TNF-α) [105]. These findings suggest a direct relationship between the anti-inflammatory abilities of terpenes and TLR-mediated pathways involved in CVDs.
These results suggest that combination therapy of taurine and black pepper terpenes may hold therapeutic cardiovascular-protective value for attenuating inflammation through modulation of TLR2 and 4 signaling pathways, which play a significant role in the development of CVDs.
Table 1.
Summary of anti-inflammatory effects of taurine and terpenes in cell and animal models.
| Compound | Dosage | Experimental Model | Outcome | Ref |
|---|---|---|---|---|
| α-humulene | 50 mg/kg | In vivo LPS-induced inflammation in the paw of Wistar rats | ↓ neutrophil migration, ↓ IL-5 ↓ NF-κB DNA binding ↓ IL-1β and TNF-α expression. |
[106] |
| α-humulene | 50 mg/kg, 22 days |
In vivo female BALB/c mice | ↓ Eosinophil recruitment ↓ NF-κB and activator protein-1 activation |
[107] |
| α-pinene | 50 mg/kg, 7 days |
In vivo ISO-induced myocardial inflammation in Wistar albino rats | ↓ cardiac injury biomarkers ↓ NF-κB signaling ↓ IL-6 and TNF-α expression |
[108] |
| BCP | 50–200 µg/mL | In vitro Mouse RAW267.4 macrophages | ↓ ERK/p38-MAPK signaling ↓ COX-1 and COX-2 |
[109] |
| BCP | (0.2–25 µM) | In vitro LPS-induced inflammation in C57BL/6 mouse microglial cells | ↓ IL-1β, TNF-α, PGE2, iNOS expression ↓ ROS inflammatory biomarkers |
[110] |
| BCP | 10 mg/kg | In vivo cisplatin-induced nephropathy in C57BL/6J mice | CB2R-dependant decrease in MCP-1, IL-1β, TNF-α, ICAM-1, neutrophil and macrophage infiltration | [111] |
| Limonene | 50 mg/kg, 21 days |
In vivo ISO-induced inflammation in male Wister rats | ↓ MAPK/JNK/ERK/NF-κB signaling ↓ IL-1β, IL-6, and TNF-α expression |
[112] |
| Limonene | 20, 50, and 100 mg/kg | In vivo gastritis-induced male Sprague-Dawley rats | ↓ NF-κB nuclear translocation ↓ intracellular Ca2+, IL-1β, IL-6, TNF |
[113] |
| Sabinene | 0.32–1.25 µL/mL, 1 h |
In vitro LPS-induced inflammation in mouse Raw 264.7 leukemic macrophage cell line | Strong anti-inflammatory activity through potent NO scavenging and inhibition of iNOS | [114] |
| Taurine | 100 mM, 30 days |
In vivo myocardial ischemia-induced male albino Wister rats | ↓ myocardial infarct size ↑ superoxide dismutase ↓ IL-6 and TNF-α expression |
[44] |
| Taurine | 3000 mg/day, 8 weeks |
Clinical study of 50 patients with type-2 diabetes | ↓ TNF-α ↑ superoxide dismutase ↑ catalase |
[115] |
Abbreviations: BCP, β-caryophyllene; Ca2+, calcium; CB2R, cannabinoid 2 receptor; COX, cyclooxygenase; ERK, extracellular signal-regulated kinase; ICAM-1, intercellular adhesion molecule 1; IκKβ, nuclear factor kappa-B kinase subunit beta; IL, interleukin; iNOS, inducible nitric oxide synthase; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein-1; NF-κB, nuclear factor kappa-light chain enhancer of activated B cells; NO, nitric oxide; PGE2, prostaglandin 2; ROS, reactive oxygen species; TNF-α, tumor necrosis factor alpha; ↑, increase; ↓, decrease.
References
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory effects of dietary polyphenols. Nutrients 2021, 13, 728.
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis Terpenes. Molecules 2020, 25, 5792.
- Parashar, S.; Singh, S.; Sood, G. Examining the role of health consciousness, environmental awareness and intention on purchase of organic food: A moderated model of attitude. J. Clean. Prod. 2023, 386, 135553.
- Cho, K.S.; Lim, Y.R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106.
- Ooi, B.K.; Chan, K.G.; Goh, B.H.; Yap, W.H. The Role of Natural Products in Targeting Cardiovascular Diseases via Nrf2 Pathway: Novel Molecular Mechanisms and Therapeutic Approaches. Front. Pharmacol. 2018, 9, 1308.
- Prakash, M.D.; Fraser, S.; Boer, J.C.; Plebanski, M.; de Courten, B.; Apostolopoulos, V. Anti-cancer effects of carnosine—A dipeptide molecule. Molecules 2021, 26, 1644.
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nothlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527.
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988.
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021.
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639.
- Mendis, S.; Davis, S.; Norrving, B. Organizational update: The world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 2015, 46, e121–e122.
- Lusis, A.J.; Fogelman, A.M.; Fonarow, G.C. Genetic basis of atherosclerosis: Part I: New genes and pathways. Circulation 2004, 110, 1868–1873.
- Dimmeler, S. Cardiovascular disease review series. EMBO Mol. Med. 2011, 3, 697.
- Bosevski, M.; Nikolovski, P.; Stojanovska, L.; Apostolopoulos, V. Progression of carotid artery disease could stratify a risk of coronary artery disease patients with type 2 diabetes. Acta Biochim. Biophys. Sin. 2019, 51, 120–122.
- Islam, S.U.; Ahmed, M.B.; Ahsan, H.; Lee, Y.S. Recent Molecular Mechanisms and Beneficial Effects of Phytochemicals and Plant-Based Whole Foods in Reducing LDL-C and Preventing Cardiovascular Disease. Antioxidants 2021, 10, 784.
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422.
- Kumar, A.; Siddharth, V.; Singh, S.I.; Narang, R. Cost analysis of treating cardiovascular diseases in a super-specialty hospital. PLoS ONE 2022, 17, e0262190.
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front. Pharmacol. 2020, 11, 422.
- Shakoor, H.; Platat, C.; Ali, H.I.; Ismail, L.C.; Al Dhaheri, A.S.; Bosevski, M.; Apostolopoulos, V.; Stojanovska, L. The benefits of physical activity in middle-aged individuals for cardiovascular disease outcomes. Maturitas 2023, 168, 49–52.
- Apostolopoulos, V.; Borkoles, E.; Polman, R.; Stojanovska, L. Physical and immunological aspects of exercise in chronic diseases. Immunotherapy 2014, 6, 1145–1157.
- Bkaily, G.; Jazzar, A.; Normand, A.; Simon, Y.; Al-Khoury, J.; Jacques, D. Taurine and cardiac disease: State of the art and perspectives. Can. J. Physiol. Pharmacol. 2020, 98, 67–73.
- Xu, Y.J.; Arneja, A.S.; Tappia, P.S.; Dhalla, N.S. The potential health benefits of taurine in cardiovascular disease. Exp. Clin. Cardiol. 2008, 13, 57–65.
- Obeid, O.A.; Johnston, K.; Emery, P.W. Plasma taurine and cysteine levels following an oral methionine load: Relationship with coronary heart disease. Eur. J. Clin. Nutr. 2004, 58, 105–109.
- Zulli, A. Taurine in cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 57–60.
- Qaradakhi, T.; Gadanec, L.K.; McSweeney, K.R.; Abraham, J.R.; Apostolopoulos, V.; Zulli, A. The Anti-Inflammatory Effect of Taurine on Cardiovascular Disease. Nutrients 2020, 12, 2847.
- Wang, D.; Zhang, L.; Huang, J.; Himabindu, K.; Tewari, D.; Horbańczuk, J.O.; Xu, S.; Chen, Z.; Atanasov, A.G. Cardiovascular protective effect of black pepper (Piper nigrum L.) and its major bioactive constituent piperine. Trends Food Sci. Technol. 2021, 117, 34–45.
- Musenga, A.; Mandrioli, R.; Ferranti, A.; D’Orazio, G.; Fanali, S.; Raggi, M.A. Analysis of aromatic and terpenic constituents of pepper extracts by capillary electrochromatography. J. Sep. Sci. 2007, 30, 612–619.
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710.
- Dosoky, N.S.; Satyal, P.; Barata, L.M.; da Silva, J.K.R.; Setzer, W.N. Volatiles of black pepper fruits (Piper nigrum L.). Molecules 2019, 24, 4244.
- Steffens, S.; Pacher, P. Targeting cannabinoid receptor CB2 in cardiovascular disorders: Promises and controversies. Br. J. Pharmacol. 2012, 167, 313–323.
- Finlay, D.B.; Sircombe, K.J.; Nimick, M.; Jones, C.; Glass, M. Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front. Pharmacol. 2020, 11, 359.
- Santiago, M.; Sachdev, S.; Arnold, J.C.; McGregor, I.S.; Connor, M. Absence of entourage: Terpenoids commonly found in Cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannabis Cannabinoid Res. 2019, 4, 165–176.
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104.
- Donkor, P.O.; Chen, Y.; Ding, L.; Qiu, F. Locally and traditionally used Ligusticum species—A review of their phytochemistry, pharmacology and pharmacokinetics. J. Ethnopharmacol. 2016, 194, 530–548.
- Suroowan, S.; Mahomoodally, F. Common phyto-remedies used against cardiovascular diseases and their potential to induce adverse events in cardiovascular patients. Clin. Phytosci. 2015, 1, 1.
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019, 20, 3879.
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906.
- Ko, Y.J.; Ahn, G.; Ham, Y.M.; Song, S.M.; Ko, E.Y.; Cho, S.H.; Yoon, W.J.; Kim, K.N. Anti-inflammatory effect and mechanism of action of Lindera erythrocarpa essential oil in lipopolysaccharide-stimulated RAW264.7 cells. EXCLI J. 2017, 16, 1103–1113.
- Li, F.; Zhang, J.; Lin, M.; Su, X.; Li, C.; Wang, H.; Li, B.; Chen, R.; Kang, J. Anti-inflammatory terpenes from Schefflera rubriflora C. J. Tseng & G. Hoo with their TNF-alpha and IL-6 inhibitory activities. Phytochemistry 2019, 163, 23–32.
- Yoon, W.J.; Lee, N.H.; Hyun, C.G. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J. Oleo Sci. 2010, 59, 415–421.
- Sun, Q.; Hu, H.; Wang, W.; Jin, H.; Feng, G.; Jia, N. Taurine attenuates amyloid beta 1-42-induced mitochondrial dysfunction by activating of SIRT1 in SK-N-SH cells. Biochem. Biophys. Res. Commun. 2014, 447, 485–489.
- Su, Y.; Fan, W.; Ma, Z.; Wen, X.; Wang, W.; Wu, Q.; Huang, H. Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience 2014, 266, 56–65.
- Niu, X.; Zheng, S.; Liu, H.; Li, S. Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Mol. Med. Rep. 2018, 18, 4516–4522.
- Hou, X.; Sun, G.; Guo, L.; Gong, Z.; Han, Y.; Bai, X. Cardioprotective effect of taurine and β-alanine against cardiac disease in myocardial ischemia and reperfusion-induced rats. Electron. J. Biotechnol. 2020, 45, 46–52.
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2014, 46, 7–20.
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol. Cells 2003, 16, 97–105.
- Solt, L.A.; May, M.J. The IkappaB kinase complex: Master regulator of NF-kappaB signaling. Immunol. Res. 2008, 42, 3–18.
- Rajapakse, N.; Kim, M.M.; Mendis, E.; Kim, S.K. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264.7 cells by carboxybutyrylated glucosamine takes place via down-regulation of mitogen-activated protein kinase-mediated nuclear factor-kappaB signaling. Immunology 2008, 123, 348–357.
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023.
- Tobon-Velasco, J.C.; Cuevas, E.; Torres-Ramos, M.A. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol. Disord. Drug Targets 2014, 13, 1615–1626.
- Chauhan, A.; Islam, A.U.; Prakash, H.; Singh, S. Phytochemicals targeting NF-kappaB signaling: Potential anti-cancer interventions. J. Pharm. Anal. 2022, 12, 394–405.
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737.
- Hsieh, I.N.; Chang, A.S.; Teng, C.M.; Chen, C.C.; Yang, C.R. Aciculatin inhibits lipopolysaccharide-mediated inducible nitric oxide synthase and cyclooxygenase-2 expression via suppressing NF-kappaB and JNK/p38 MAPK activation pathways. J. Biomed. Sci. 2011, 18, 28.
- Ham, Y.-M.; Ko, Y.-J.; Song, S.-M.; Kim, J.; Kim, K.-N.; Yun, J.-H.; Cho, J.-H.; Ahn, G.; Yoon, W.-J. Anti-inflammatory effect of litsenolide B2 isolated from Litsea japonica fruit via suppressing NF-κB and MAPK pathways in LPS-induced RAW264.7 cells. J. Funct. Foods 2015, 13, 80–88.
- Del Prado-Audelo, M.L.; Cortes, H.; Caballero-Floran, I.H.; Gonzalez-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chavez, S.A.; Giraldo-Gomez, D.M.; Magana, J.J.; Leyva-Gomez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197.
- Barua, M.; Liu, Y.; Quinn, M.R. Taurine chloramine inhibits inducible nitric oxide synthase and TNF-alpha gene expression in activated alveolar macrophages: Decreased NF-kappaB activation and IkappaB kinase activity. J. Immunol. 2001, 167, 2275–2281.
- Kim, B.S.; Cho, I.S.; Park, S.Y.; Schuller-Levis, G.; Levis, W.; Park, E. Taurine chloramine inhibits NO and TNF-alpha production in zymosan plus interferon-gamma activated RAW 264.7 cells. J. Drugs Dermatol. 2011, 10, 659–665.
- Kim, J.W.; Kim, C. Inhibition of LPS-induced NO production by taurine chloramine in macrophages is mediated though Ras-ERK-NF-kappaB. Biochem. Pharmacol. 2005, 70, 1352–1360.
- Chi, G.; Wei, M.; Xie, X.; Soromou, L.W.; Liu, F.; Zhao, S. Suppression of MAPK and NF-kappaB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation 2013, 36, 501–511.
- Nam, S.Y.; Chung, C.K.; Seo, J.H.; Rah, S.Y.; Kim, H.M.; Jeong, H.J. The therapeutic efficacy of alpha-pinene in an experimental mouse model of allergic rhinitis. Int. Immunopharmacol. 2014, 23, 273–282.
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-alpha-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269.
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150.
- Yang, J.; Choi, W.S.; Kim, K.J.; Eom, C.D.; Park, M.J. Investigation of Active Anti-Inflammatory Constituents of Essential Oil from Pinus koraiensis (Sieb. et Zucc.) Wood in LPS-Stimulated RBL-2H3 Cells. Biomolecules 2021, 11, 817.
- Aly, E.; Khajah, M.A.; Masocha, W. beta-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules 2019, 25, 106.
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB(2) receptor and its role as a regulator of inflammation. Cell Mol. Life Sci. 2016, 73, 4449–4470.
- Benicky, J.; Sanchez-Lemus, E.; Pavel, J.; Saavedra, J.M. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol. Neurobiol. 2009, 29, 781–792.
- Parlar, A.; Arslan, S.O.; Dogan, M.F.; Cam, S.A.; Yalcin, A.; Elibol, E.; Ozer, M.K.; Uckardes, F.; Kara, H. The exogenous administration of CB2 specific agonist, GW405833, inhibits inflammation by reducing cytokine production and oxidative stress. Exp. Ther. Med. 2018, 16, 4900–4908.
- Guo, K.; Mou, X.; Huang, J.; Xiong, N.; Li, H. Trans-caryophyllene suppresses hypoxia-induced neuroinflammatory responses by inhibiting NF-kappaB activation in microglia. J. Mol. Neurosci. 2014, 54, 41–48.
- Meeran, M.N.; Laham, F.; Azimullah, S.; Sharma, C.; Al Kaabi, A.J.; Tariq, S.; Adeghate, E.; Goyal, S.N.; Ojha, S. β-Caryophyllene, a natural bicyclic sesquiterpene attenuates β-adrenergic agonist-induced myocardial injury in a cannabinoid receptor-2 dependent and independent manner. Free Radic Biol. Med. 2021, 167, 348–366.
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of CB2 and PPAR-gamma receptors. Chem. Biol. Interact. 2019, 297, 16–24.
- Zhang, Z.; Yang, C.; Dai, X.; Ao, Y.; Li, Y. Inhibitory effect of trans-caryophyllene (TC) on leukocyte-endothelial attachment. Toxicol. Appl. Pharmacol. 2017, 329, 326–333.
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328.
- Tong, Y.; Wang, Z.; Cai, L.; Lin, L.; Liu, J.; Cheng, J. NLRP3 Inflammasome and Its Central Role in the Cardiovascular Diseases. Oxid Med. Cell Longev. 2020, 2020, 4293206.
- Hortelano, S.; Gonzalez-Cofrade, L.; Cuadrado, I.; de Las Heras, B. Current status of terpenoids as inflammasome inhibitors. Biochem. Pharmacol. 2020, 172, 113739.
- Lee, C.C.; Chen, W.T.; Chen, S.Y.; Lee, T.M. Taurine Alleviates Sympathetic Innervation by Inhibiting NLRP3 Inflammasome in Postinfarcted Rats. J. Cardiovasc. Pharmacol. 2021, 77, 745–755.
- Qiu, T.; Pei, P.; Yao, X.; Jiang, L.; Wei, S.; Wang, Z.; Bai, J.; Yang, G.; Gao, N.; Yang, L.; et al. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis. 2018, 9, 946.
- Zou, X.S.; Xie, L.; Wang, W.Y.; Zhao, G.Y.; Tian, X.Y.; Chen, M.H. Pomelo peel oil alleviates cerebral NLRP3 inflammasome activation in a cardiopulmonary resuscitation rat model. Exp. Ther. Med. 2021, 21, 233.
- Wong, W.T.; Wu, C.H.; Li, L.H.; Hung, D.Y.; Chiu, H.W.; Hsu, H.T.; Ho, C.L.; Chernikov, O.V.; Cheng, S.M.; Yang, S.P.; et al. The leaves of the seasoning plant Litsea cubeba inhibit the NLRP3 inflammasome and ameliorate dextran sulfate sodium-induced colitis in mice. Front. Nutr. 2022, 9, 871325.
- Yu, W.; Jin, G.; Zhang, J.; Wei, W. Selective Activation of Cannabinoid Receptor 2 Attenuates Myocardial Infarction via Suppressing NLRP3 Inflammasome. Inflammation 2019, 42, 904–914.
- Yuan, S.; Liu, Z.; Xu, Z.; Liu, J.; Zhang, J. High mobility group box 1 (HMGB1): A pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 2020, 13, 1–19.
- Tang, D.; Kang, R.; Livesey, K.M.; Kroemer, G.; Billiar, T.R.; Van Houten, B.; Zeh Iii, H.J.; Lotze, M.T. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011, 13, 701–711.
- Matsumiya, M.; Stylianou, E.; Griffiths, K.; Lang, Z.; Meyer, J.; Harris, S.A.; Rowland, R.; Minassian, A.M.; Pathan, A.A.; Fletcher, H. Roles for Treg expansion and HMGB1 signaling through the TLR1-2-6 axis in determining the magnitude of the antigen-specific immune response to MVA85A. PLoS ONE 2013, 8, e67922.
- Andersson, U.; Erlandsson-Harris, H.; Yang, H.; Tracey, K.J. HMGB1 as a DNA-binding cytokine. J. Leukoc. Biol. 2002, 72, 1084–1091.
- Wahid, A.; Chen, W.; Wang, X.; Tang, X. High-mobility group box 1 serves as an inflammation driver of cardiovascular disease. Biomed. Pharmacother. 2021, 139, 111555.
- Yan, X.X.; Lu, L.; Peng, W.H.; Wang, L.J.; Zhang, Q.; Zhang, R.Y.; Chen, Q.J.; Shen, W.F. Increased serum HMGB1 level is associated with coronary artery disease in nondiabetic and type 2 diabetic patients. Atherosclerosis 2009, 205, 544–548.
- Hu, X.; Jiang, H.; Bai, Q.; Zhou, X.; Xu, C.; Lu, Z.; Cui, B.; Wen, H. Increased serum HMGB1 is related to the severity of coronary artery stenosis. Clin. Chim. Acta 2009, 406, 139–142.
- Giovannini, S.; Tinelli, G.; Biscetti, F.; Straface, G.; Angelini, F.; Pitocco, D.; Mucci, L.; Landolfi, R.; Flex, A. Serum high mobility group box-1 and osteoprotegerin levels are associated with peripheral arterial disease and critical limb ischemia in type 2 diabetic subjects. Cardiovasc. Diabetol. 2017, 16, 99.
- Ma, Y.; Zhang, Z.; Chen, R.; Shi, R.; Zeng, P.; Chen, R.; Leng, Y.; Chen, A.F. NRP1 regulates HMGB1 in vascular endothelial cells under high homocysteine condition. Am. J. Physiol.—Heart Circ. Physiol. 2019, 316, H1039–H1046.
- Jiang, H.; Hu, X.; Zhang, H.; Li, W. Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder cancer via suppressing HMGB1 expression. Radiat. Oncol. 2017, 12, 65.
- Gaskell, H.; Ge, X.; Desert, R.; Das, S.; Han, H.; Lantvit, D.; Guzman, G.; Nieto, N. Ablation of Hmgb1 in intestinal epithelial cells causes intestinal lipid accumulation and reduces NASH in mice. Hepatol. Commun. 2020, 4, 92–108.
- Gui, H.; Sun, Y.; Luo, Z.-M.; Su, D.-F.; Dai, S.-M.; Liu, X. Cannabinoid receptor 2 protects against acute experimental sepsis in mice. Mediat. Inflamm. 2013, 2013, 741303.
- Zhou, H.; Du, R.; Li, G.; Bai, Z.; Ma, J.; Mao, C.; Wang, J.; Gui, H. Cannabinoid receptor 2 promotes the intracellular degradation of HMGB1 via the autophagy-lysosome pathway in macrophage. Int. Immunopharmacol. 2020, 78, 106007.
- Cho, H.-I.; Hong, J.-M.; Choi, J.-W.; Choi, H.-S.; Kwak, J.H.; Lee, D.-U.; Lee, S.K.; Lee, S.-M. β-Caryophyllene alleviates d-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. Eur. J. Pharmacol. 2015, 764, 613–621.
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376.
- Frantz, S.; Ertl, G.; Bauersachs, J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 444–454.
- Jaén, R.I.; Val-Blasco, A.; Prieto, P.; Gil-Fernández, M.; Smani, T.; López-Sendón, J.L.; Delgado, C.; Boscá, L.; Fernández-Velasco, M. Innate immune receptors, key actors in cardiovascular diseases. Basic Transl. Sci. 2020, 5, 735–749.
- Zhao, D.; Zhang, X.; Feng, Y.; Bian, Y.; Fu, Z.; Wu, Y.; Ma, Y.; Li, C.; Wang, J.; Dai, J. Taurine Alleviates LPS-Induced Acute Lung Injury by Suppressing TLR-4/NF-κB Pathway. In Taurine 12: A Conditionally Essential Amino Acid; Springer: Berlin/Heidelberg, Germany, 2022; pp. 63–72.
- Lin, C.-J.; Chiu, C.-C.; Chen, Y.-C.; Chen, M.-L.; Hsu, T.-C.; Tzang, B.-S. Taurine attenuates hepatic inflammation in chronic alcohol-fed rats through inhibition of TLR4/MyD88 signaling. J. Med. Food 2015, 18, 1291–1298.
- Younis, N.S.; Ghanim, A.M.; Elmorsy, M.A.; Metwaly, H.A. Taurine ameliorates thioacetamide induced liver fibrosis in rats via modulation of toll like receptor 4/nuclear factor kappa B signaling pathway. Sci. Rep. 2021, 11, 12296.
- Premkumar, V.; Dey, M.; Dorn, R.; Raskin, I. MyD88-dependent and independent pathways of Toll-Like Receptors are engaged in biological activity of Triptolide in ligand-stimulated macrophages. BMC Chem. Biol. 2010, 10, 3.
- Triantafilou, M.; Gamper, F.G.; Haston, R.M.; Mouratis, M.A.; Morath, S.; Hartung, T.; Triantafilou, K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 2006, 281, 31002–31011.
- Li, M.; Gao, Y.; Wang, Z.; Wu, B.; Zhang, J.; Xu, Y.; Han, X.; Phouthapane, V.; Miao, J. Taurine inhibits Streptococcus uberis-induced NADPH oxidase-dependent neutrophil extracellular traps via TAK1/MAPK signaling pathways. Front. Immunol. 2022, 13, 927215.
- Miao, J.; Zheng, L.; Zhang, J.; Ma, Z.; Zhu, W.; Zou, S. The effect of taurine on the toll-like receptors/nuclear factor kappa B (TLRs/NF-kappaB) signaling pathway in Streptococcus uberis-induced mastitis in rats. Int. Immunopharmacol. 2011, 11, 1740–1746.
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.-H. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015, 43, 731–742.
- Younis, N.S.; Mohamed, M.E. β-Caryophyllene as a potential protective agent against myocardial injury: The role of toll-like receptors. Molecules 2019, 24, 1929.
- Medeiros, R.; Passos, G.F.; Vitor, C.E.; Koepp, J.; Mazzuco, T.L.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br. J. Pharmacol. 2007, 151, 618–627.
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.; Figueiredo, C.P.; Calixto, J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene alpha-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009, 158, 1074–1087.
- Zhang, B.; Wang, H.; Yang, Z.; Cao, M.; Wang, K.; Wang, G.; Zhao, Y. Protective effect of alpha-pinene against isoproterenol-induced myocardial infarction through NF-kappaB signaling pathway. Hum. Exp. Toxicol. 2020, 39, 1596–1606.
- Yamaguchi, M.; Levy, R.M. The combination of beta-caryophyllene, baicalin and catechin synergistically suppresses the proliferation and promotes the death of RAW267.4 macrophages in vitro. Int. J. Mol. Med. 2016, 38, 1940–1946.
- Askari, V.R.; Shafiee-Nick, R. The protective effects of beta-caryophyllene on LPS-induced primary microglia M(1)/M(2) imbalance: A mechanistic evaluation. Life Sci. 2019, 219, 40–73.
- Horvath, B.; Mukhopadhyay, P.; Kechrid, M.; Patel, V.; Tanchian, G.; Wink, D.A.; Gertsch, J.; Pacher, P. beta-Caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Radic. Biol. Med. 2012, 52, 1325–1333.
- Younis, N.S. D-Limonene mitigate myocardial injury in rats through MAPK/ERK/NF-kappaB pathway inhibition. Korean J. Physiol. Pharmacol. 2020, 24, 259–266.
- Yeo, D.; Hwang, S.J.; Song, Y.S.; Lee, H.J. Humulene Inhibits Acute Gastric Mucosal Injury by Enhancing Mucosal Integrity. Antioxidants 2021, 10, 761.
- Valente, J.; Zuzarte, M.; Goncalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354.
- Maleki, V.; Mahdavi, R.; Hajizadeh-Sharafabad, F.; Alizadeh, M. The effects of taurine supplementation on oxidative stress indices and inflammation biomarkers in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Diabetol. Metab. Syndr. 2020, 12, 9.
More
