Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Agnieszka Szuster-Ciesielska.
The incidence of allergic diseases worldwide is rapidly increasing, making allergies a modern pandemic. Human activity and climate change have an impact on the spread of fungi and their plant hosts. Particular attention should be paid to microfungi, i.e., plant parasites that may be an underestimated source of new allergens.
- overreactivity-related diseases
- fungal-related allergic diseases
- microfungi
1. Introduction
Allergies are a group of complex diseases characterized by an inappropriate or overreactive immune response of the organism to common environmental substances named allergens. Most allergens, e.g., house dust mites, pollen, fungal or mold spores, food (especially milk, eggs, fruit, wheat, soybeans, seafood, and nuts), insect venoms (e.g., wasps and bees), some medications, latex, and household chemicals, are harmless to humans. Upon contact with these agents, a hypersensitivity reaction develops and antibodies are produced in predisposed subjects [1,2][1][2]. Depending on the cause, i.e., the type of contact with the allergen, the following conditions are distinguished: (1) respiratory system allergies, i.e., allergic rhinitis with conjunctivitis and asthma with wheezing, coughing, and shortness of breath; (2) contact (skin) allergies, among which the most common are atopic and contact dermatitis, manifested mainly in rashes; (3) food allergies, and (4) insect venom allergies, which cause a wide range of symptoms and can be life-threatening in some cases (anaphylaxis) [3,4,5][3][4][5].
2. Allergy Epidemiology
The incidence of allergic diseases in the world is rapidly increasing, and according to the estimates of the World Allergy Organization (WAO), it ranges from 10–40%, depending on the country. In most developed countries, allergy affects over 20% of the population [6]. While allergy was regarded as a rare disease in the early 20th century, the last few decades have witnessed a dramatic increase in its prevalence. Currently, more than 150 million Europeans suffer from chronic allergic diseases, and 20% struggle with severe and debilitating forms. By 2025, half the European Union population is estimated to suffer from allergies. The prevalence of allergic diseases is increasing together with the progress in urbanization, industrialization, pollution, and climate change, factors that are not likely to change quickly. Moreover, these data are considered to be underestimations, because many patients do not report their symptoms or are misdiagnosed, with potentially 45% of patients never receiving an allergy diagnosis [7].
Respiratory allergies are the most common allergy type in Europe and worldwide [8,9][8][9]. Data in the White Book of Allergy published in 2013 by the WAO confirm that the prevalence of allergic rhinitis and asthma is increasing worldwide [10]. Allergic rhinitis with conjunctivitis is the most common non-infectious rhinitis, affecting approximately 400 million people worldwide [11,12][11][12]. Asthma is one of the most common chronic diseases, affecting about 339 million people worldwide, and its prevalence is increasing, especially among children [13,14][13][14]. The most common asthma phenotype is allergic asthma. It is estimated that up to 89% of childhood asthma and more than 50% of adult asthma cases may have an allergic component [15]. According to the European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), allergic rhinitis affects 4–40% of Europe’s population [16]. In comparison, the prevalence of asthma in the European Union is 8.2% in adults and 9.4% in children [17]. In 2019, Portugal and Sweden were the EU countries with the highest prevalence of asthma, with approximately 9.9% and 8.2% of their populations suffering from the disease, respectively [18].
3. Types of Hypersensitivity Reactions
One of the functions of the human immune system is to provide protection against such microorganisms as bacteria, viruses, and fungi. Exaggerated immune responses and hypersensitivity reactions may lead to disease development. In 1963, Gell and Combs, who investigated the effector mechanism responsible for cell and tissue damage and the type of immune response, created the basis for the classification of hypersensitivity reactions, dividing them into four distinct types: type I (immediate or IgE-dependent), type II (cytotoxic or dependent on IgG/IgM), type III (immune complexes), and type IV (delayed or dependent on T lymphocytes) [19]. However, in clinical practice, the categories of hypersensitivity may overlap, because patients exhibit simultaneous coexistence of symptoms characteristic of specific hypersensitivity reactions [20,21][20][21].
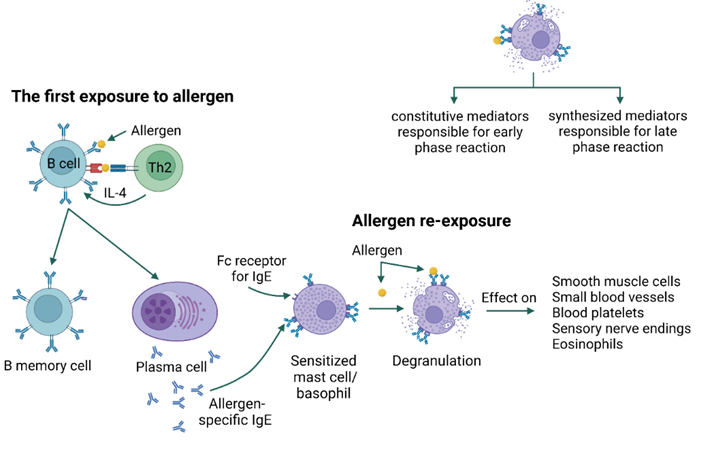
In type I hypersensitivity, type 2 helper T cells (Th2) and their mediators initiate a change in the isotype of B cells, producing allergen-specific IgE. IgE antibodies bind to receptors (FcεRI) present on the surface of mast cells and basophils [21]. Upon re-exposure, the allergen is bound by IgE, resulting in an immediate type I hypersensitivity reaction in two phases. The early phase occurs within minutes of allergen exposure and is triggered by histamine, proteases, lysosomal enzymes, and other mediators released after the degranulation of mast cells and basophils. In addition, mast cells produce lipid mediators, including prostaglandin D2 and leukotriene D4, from arachidonic acid, and release them into the circulation. The late phase begins 4 to 8 h after allergen exposure and is mediated by cytokines IL-1, IL-4, IL-5, IL-13, tumor necrosis factor (TNF-α), and granulocyte-monocyte colony-stimulating factor (GM-CSF) [21,22,23][21][22][23] (Figure 1). Immediate hypersensitivity reactions include anaphylaxis, bronchial asthma, and urticaria [21,24][21][24].
accessed on 8 April 2023].
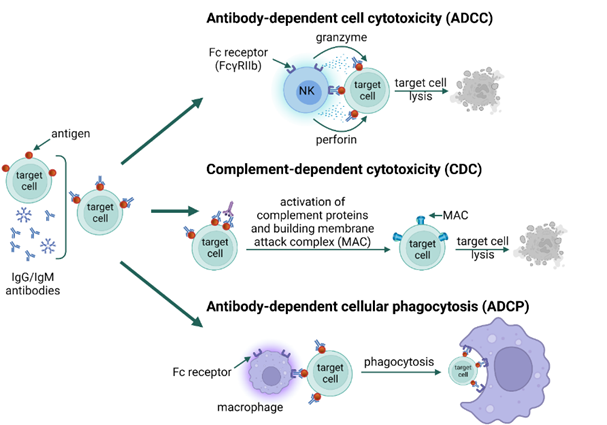
Unfortunately, antibodies can sometimes bind to self-antigens, directing a cytotoxic response against the host. This is the basis of type II hypersensitivity, a cytotoxic reaction characterized by IgG/IgM antibodies recognizing self-antigens usually found on circulating blood cells (erythrocytes, neutrophils, platelets), epithelial cells of glands and mucous membranes, or basement membranes [21]. Type II reactions are divided into two subtypes: IIa and IIb. Type IIa refers to reactions characterized by cytolytic destruction of target cells. IgG/IgM binding on the cell surface triggers cytotoxicity via three mechanisms [25]. In the first one (antibody-dependent cell cytotoxicity, ADCC), IgG binds to the Fc gamma IIb receptor (FcγRIIb) on NK cells and macrophages, which leads to their degranulation and release of perforin and granzyme directly destroying the cells. In the second mechanism (complement-dependent cytotoxicity, CDC), antigen-antibody complexes on the cell surface activate the complement via the classical pathway to form the C5–C9 membrane attack complex (MAC), which induces lysis of the target cell. The third mechanism (antibody-dependent cellular phagocytosis, ADCP) initiates phagocytosis by binding IgG and IgM to Fc receptors on phagocytes and activating them (Figure 2). Type IIb reactions refer to the direct stimulation of cells by autoantibodies and the development of disease; for example, in Graves’ disease, antibodies directed against thyrotropin receptors stimulate the thyroid gland to produce excessive amounts of the hormone [27,28][27][28].
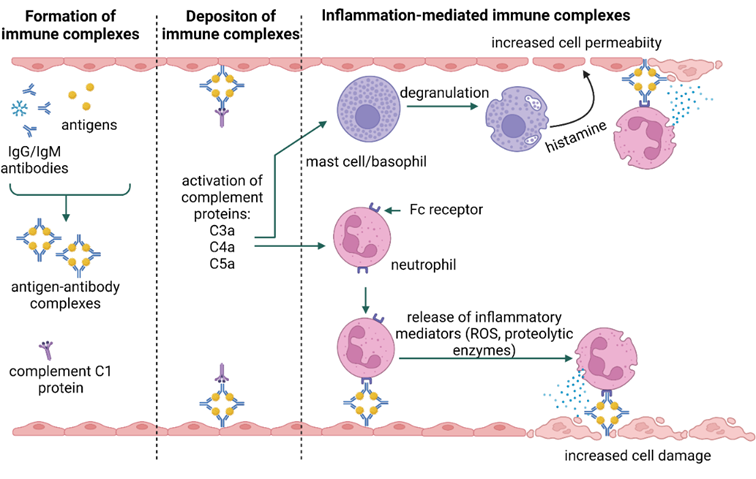
In the type III immune response, class G and M immunoglobulins bind to antigens, forming immune complexes that, deposited in tissues, activate complement system components. This leads to the recruitment and activation of neutrophils, mast cells, and basophils, which cause inflammation and tissue damage [21,25][21][25] (Figure 3). The type and nature of symptoms occurring in type III reactions depend on the site of deposition of immune complexes rather than on the source of the allergen [27,31][27][31]. The antigens involved in the type III response may be identified as self, as in the case of autoimmune diseases (e.g., lupus), or non-self [27].
accessed on 8 April 2023].
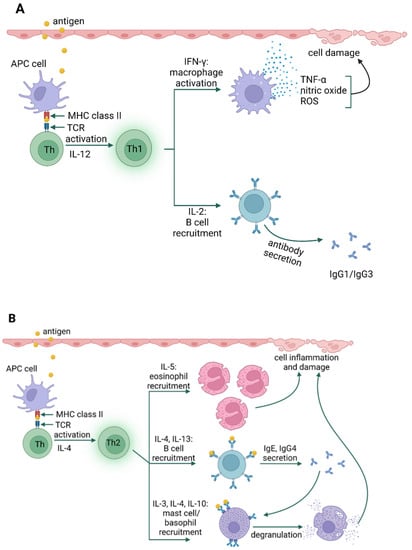
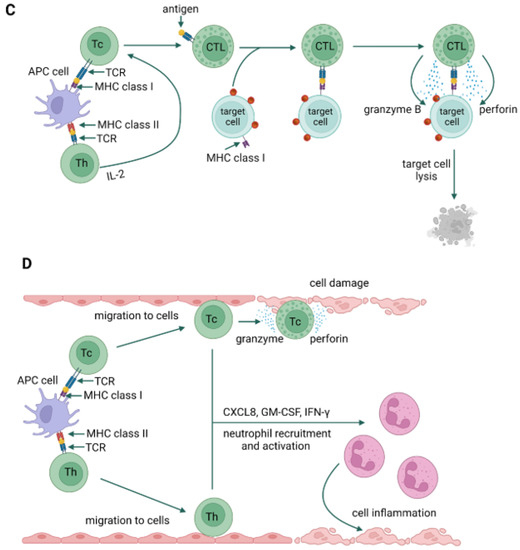
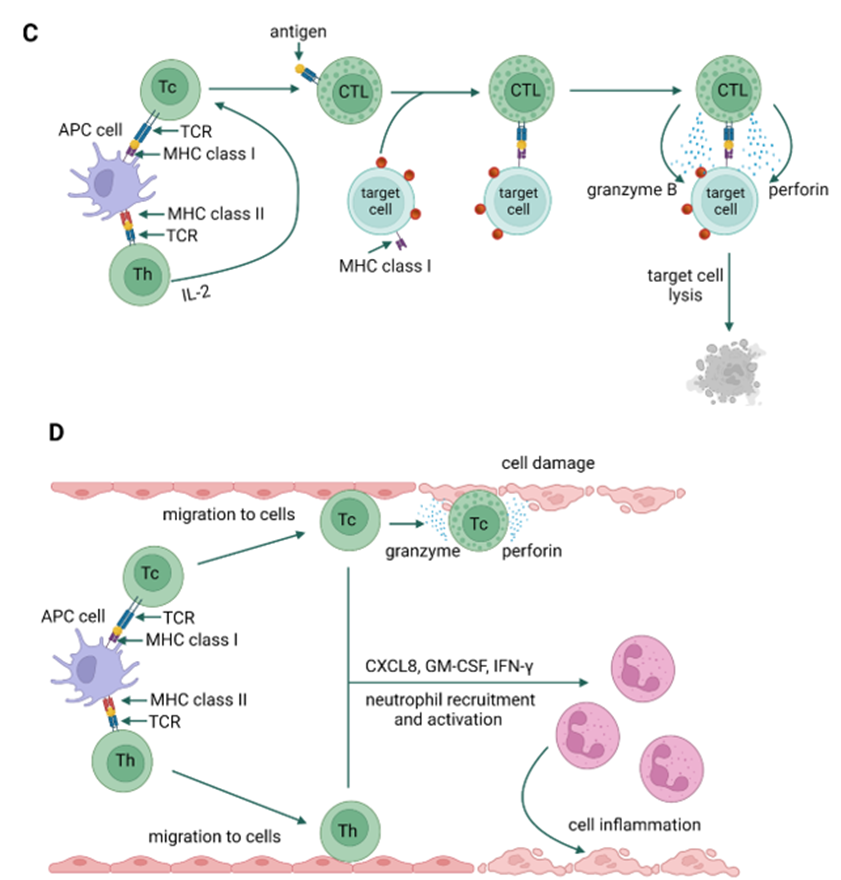
Type IV hypersensitivity reactions are referred to as delayed reactions. The primary effector cells are T lymphocytes that can cause injury directly (cytotoxic T lymphocytes, Tc) or activate other leukocytes (macrophages, neutrophils, and eosinophils) to damage tissues by releasing reactive oxygen species, lysosomal enzymes, and inflammatory cytokines (helper T cells) [33]. The relatively recent identification of T cell subsets has allowed the categorization of type IV hypersensitivity reactions into four subtypes, based on the type of cells involved, pathogenesis, and cytokine profile [21]. Type IVa is a reaction involving type 1 helper T cells (Th1), which activate and stimulate macrophages to produce such cytokines as interferon γ (IFN-γ) and TNF-α (e.g., contact dermatitis) (Figure 4A). In type IVb reactions, Th2 cells produce IL-4, IL-5, and IL-13, which initiate the production of IgE by B lymphocytes and the inactivation of macrophages (e.g., DRESS syndrome) [34]. Moreover, reactions of this type may also be involved in the late phase of allergic bronchitis or rhinitis (i.e., asthma and allergic rhinitis) (Figure 4B). IVc hypersensitivity is mainly mediated by Tc-released mediators, such as perforins, granulysin, and granzyme B to kill target cells (e.g., Stevens-Johnson syndrome) (Figure 4C). Type IVd reactions cause tissue damage due to the production of CXCL-8 (IL-8) by T lymphocytes, which promotes the recruitment of neutrophils to sites of inflammation (e.g., Behçet’s disease) (Figure 4D) [27,33,35][27][33][35].
References
- Del Moral, M.G.; Martinez-Naves, E. The Role of Lipids in Development of Allergic Responses. Immune Netw. 2017, 17, 133–143.
- Justiz Vaillant, A.A.; Vashisht, R.; Zito, P.M. Immediate Hypersensitivity Reactions; StatPearls: Treasure Island, FL, USA, 2023.
- Aldakheel, F.M. Allergic Diseases: A Comprehensive Review on Risk Factors, Immunological Mechanisms, Link with COVID-19, Potential Treatments, and Role of Allergen Bioinformatics. Int. J. Environ. Res. Public Health 2021, 18, 12105.
- Mohd Adnan, K. A review on Respiratory allergy caused by insects. Bioinformation 2018, 14, 540–553.
- Xu, G.; Liu, B.; Yang, W.; Snetselaar, L.G.; Chen, M.; Bao, W.; Strathearn, L. Association of Food Allergy, Respiratory Allergy, and Skin Allergy with Attention Deficit/Hyperactivity Disorder among Children. Nutrients 2022, 14, 474.
- Global Initiative for Asthma. Pocket Guide for Asthma Management and Prevention. 2022. Available online: https://kinderpneumologie.ch/wp-content/uploads/2022/11/gina-2022-pocket-guide-wms.pdf (accessed on 4 April 2023).
- (EAACI) Tackling the Allergy Crisis in Europe—Concerted Policy Action Needed 2015. Available online: https://www.veroval.info/-/media/diagnostics/files/knowledge/eaaci_advocacy_manifesto.pdf (accessed on 4 April 2023).
- Payandeh, P.; Fadaee, J.; Jabbari Azad, F.; Bakhshaii, M.; Sistani, S. Allergens Prevalence among Patients with Respiratory Allergies in Mashhad, Iran. Tanaffos 2019, 18, 133–141.
- Mazur, M.; Czarnobilska, M.; Dyga, W.; Czarnobilska, E. Trends in the Epidemiology of Allergic Diseases of the Airways in Children Growing Up in an Urban Agglomeration. J. Clin. Med. 2022, 11, 2188.
- AO White Book on Allergy 2013 Update. Available online: https://www.worldallergy.org/wao-white-book-on-allergy (accessed on 4 April 2023).
- Compalati, E.; Ridolo, E.; Passalacqua, G.; Braido, F.; Villa, E.; Canonica, G.W. The link between allergic rhinitis and asthma: The united airways disease. Expert. Rev. Clin. Immunol. 2010, 6, 413–423.
- Nur Husna, S.M.; Tan, H.T.; Md Shukri, N.; Mohd Ashari, N.S.; Wong, K.K. Allergic Rhinitis: A Clinical and Pathophysiological Overview. Front. Med. 2022, 9, 874114.
- 2020. Available online: https://www.who.int/news-room/facts-in-pictures/detail/asthma (accessed on 4 April 2023).
- 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 4 April 2023).
- Lieberman, P.; Nicklas, R.A.; Randolph, C.; Oppenheimer, J.; Bernstein, D.; Bernstein, J.; Ellis, A.; Golden, D.B.; Greenberger, P.; Kemp, S.; et al. Anaphylaxis—A practice parameter update 2015. Ann. Allergy Asthma Immunol. 2015, 115, 341–384.
- 2023. Available online: https://www.efanet.org/inform/patient-evidence/rhinitis (accessed on 4 April 2023).
- Selroos, O.; Kupczyk, M.; Kuna, P.; Łacwik, P.; Bousquet, J.; Brennan, D.; Palkonen, S.; Contreras, J.; Fitzgerald, M.; Hedlin, G.; et al. National and regional asthma programmes in Europe. Eur. Respir. Rev. 2015, 24, 474–483.
- 2019. Available online: https://www.statista.com/statistics/1296610/asthma-prevalence-in-the-eu/ (accessed on 4 April 2023).
- Celik, G.E.P.W.; Adkinson, N.F., Jr. Drug allergy. In Middleton’s Allergy: Principles and Practice; Adkinson, N.F., Jr., Bochner, B.S., Burks, A.W., Busse, W.W., Holgate, S.T., Lemanske, R.F., O’Hehir, R.E., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2014.
- Chinen, J.F.T.; Shearer, W.T. The Immune system: An overview. In Middleton’s Allergy Principles & Practice; Szczeklik, A., Nizankowska-Mogilnicka, E., Sanak, M., Adkinson, N.F., Bochner, B.S., Busse, W.W., Holgate, S.T., Lemanske, R.F., Simons, F.E.R., Eds.; Mosby: Philadelphia, PA, USA, 2009; pp. 3–17.
- Abbas, M.; Moussa, M.; Akel, H. Type I Hypersensitivity Reaction; StatPearls: Treasure Island, FL, USA, 2023.
- León, B.; Ballesteros-Tato, A. Modulating Th2 Cell Immunity for the Treatment of Asthma. Front. Immunol. 2021, 12, 637948.
- Pritchard, D.I.; Falcone, F.H.; Mitchell, P.D. The evolution of IgE-mediated type I hypersensitivity and its immunological value. Allergy 2021, 76, 1024–1040.
- Gulsen, A.; Wedi, B.; Jappe, U. Hypersensitivity reactions to biologics (part I): Allergy as an important differential diagnosis in complex immune-derived adverse events. Allergo J. Int. 2020, 29, 97–125.
- Dispenza, M.C. Classification of hypersensitivity reactions. In Allergy & Asthma Proceedings; OceanSide Publications, Inc.: Cocoa Beach, FL, USA, 2019; Volume 40, pp. 470–473.
- Barnes, P.J. Pathophysiology of allergic inflammation. Immunol. Rev. 2011, 242, 31–50.
- Uzzaman, A.; Cho, S.H. Chapter 28: Classification of hypersensitivity reactions. In Allergy & Asthma Proceedings; OceanSide Publications, Inc.: Cocoa Beach, FL, USA, 2012; Volume 33, (Suppl. 1), pp. 96–99.
- Carr, T.F.; Saltoun, C.A. Chapter 21: Urticaria and angioedema. In Allergy & Asthma Proceedings; OceanSide Publications, Inc.: Cocoa Beach, FL, USA, 2012; Volume 33, (Suppl. 1), pp. 70–72.
- van De Donk, N.W.C.J.; Moreau, P.; Plesner, T.; Palumbo, A.; Gay, F.; Laubach, J.P.; Malavasi, F.; Avet-Loiseau, H.; Mateos, M.-V.; Sonneveld, P.; et al. Clinical efficacy and management of monoclonal antibodies targeting CD38 and SLAMF7 in multiple myeloma. Blood 2016, 127, 681–695.
- Ballanti, E.; Perricone, C.; Greco, E.; Ballanti, M.; Di Muzio, G.; Chimenti, M.S.; Perricone, R. Complement and autoimmunity. Immunol. Res. 2013, 56, 477–491.
- Abbas, A.K.L.A.; Pillai, S. (Eds.) Immediate hypersensitivity. In Cellular and Molecular Immunology, 6th ed.; Saunders: Philadelphia, PA, USA, 2007; pp. 441–461.
- Usman, N.; Annamaraju, P. Type III Hypersensitivity Reaction; StatPearls: Treasure Island, FL, USA, 2023.
- Marwa, K.; Kondamudi, N.P. Type IV Hypersensitivity Reaction; StatPearls: Treasure Island, FL, USA, 2023.
- Kang, S.-Y.; Kim, J.; Ham, J.; Cho, S.-H.; Kang, H.-R.; Kim, H.Y. Altered T cell and monocyte subsets in prolonged immune reconstitution inflammatory syndrome related with DRESS (drug reaction with eosinophilia and systemic symptoms). Asia Pac. Allergy 2020, 10, e2.
- Pichler, W.J. Delayed drug hypersensitivity reactions. Ann. Intern. Med. 2003, 139, 683–693.
- Romagnani, S. Th1/Th2 Cells. Inflamm. Bowel Dis. 1999, 5, 285–294.
- Liu, Y.; Ng, K.-Y.; Lillehei, K.O. Cell-Mediated Immunotherapy: A New Approach to the Treatment of Malignant Glioma. Cancer Control. 2003, 10, 138–147.
- Feldmeyer, L.; Heidemeyer, K.; Yawalkar, N. Acute Generalized Exanthematous Pustulosis: Pathogenesis, Genetic Background, Clinical Variants and Therapy. Int. J. Mol. Sci. 2016, 17, 1214.
More