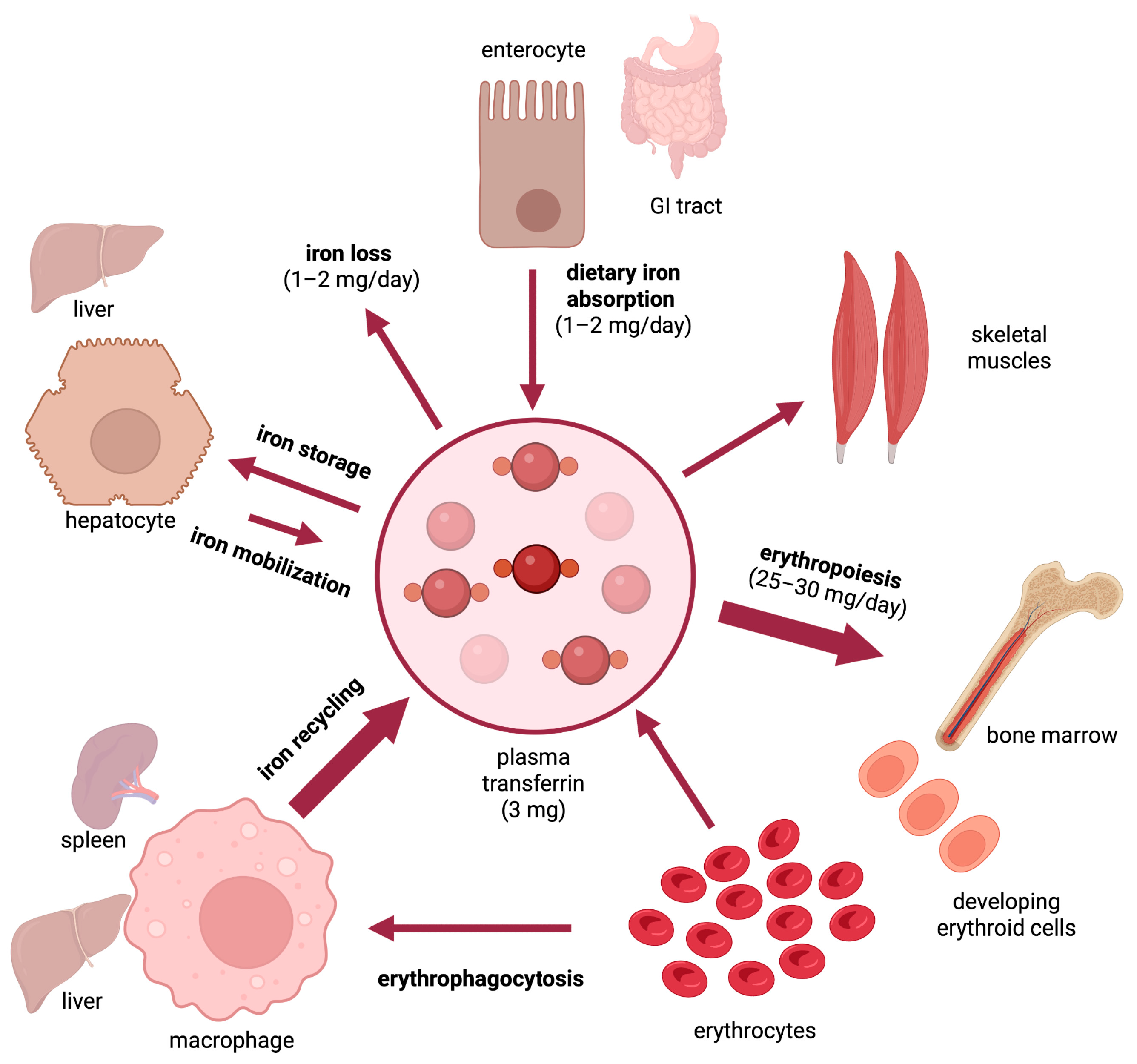
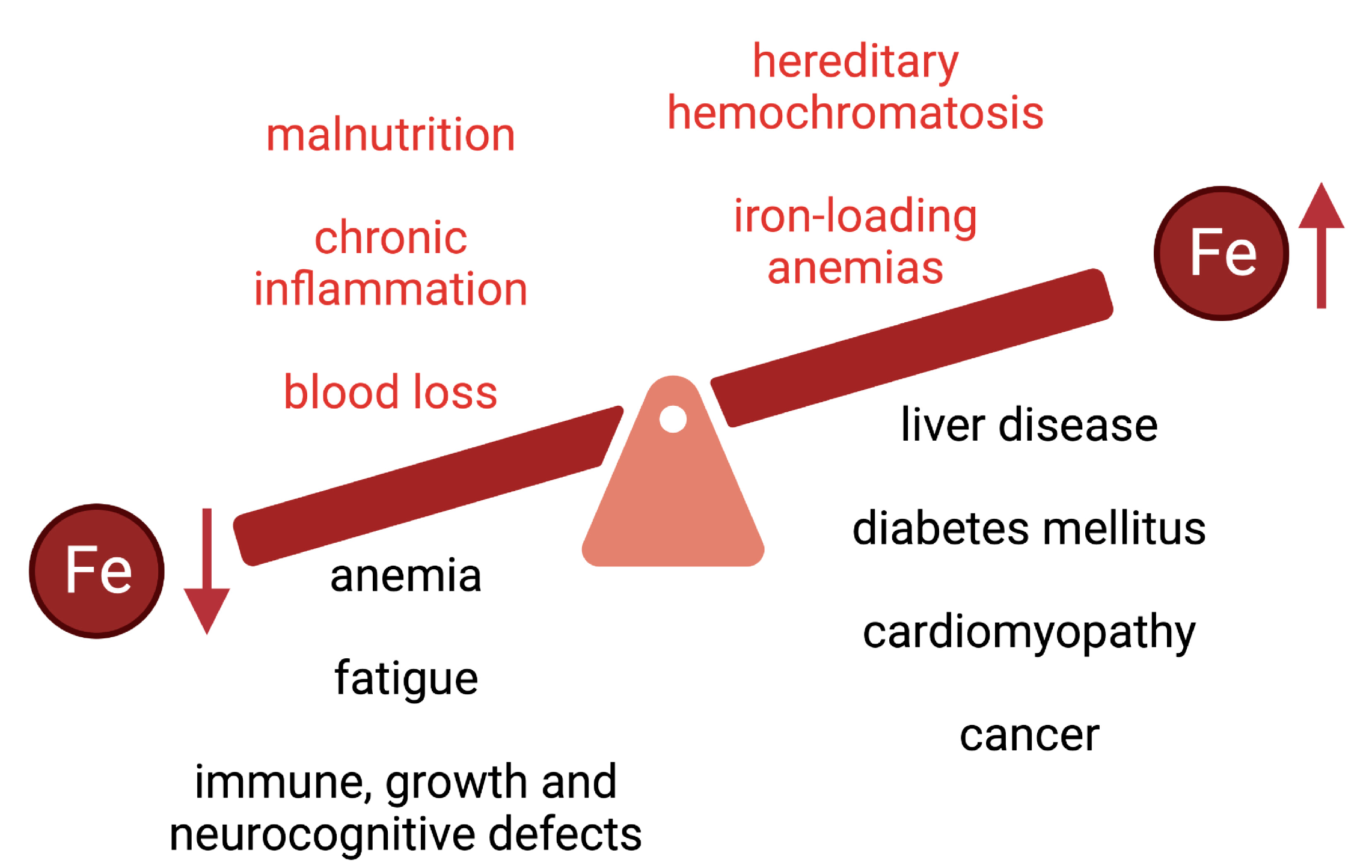
Dietary iron assimilation is critical for health and essential to prevent iron-deficient states and related comorbidities, such as anemia. The bioavailability of iron is generally low, while its absorption and metabolism are tightly controlled to satisfy metabolic needs and prevent toxicity of excessive iron accumulation. Iron entry into the bloodstream is limited by hepcidin, the iron regulatory hormone. Hepcidin deficiency due to loss-of-function mutations in upstream gene regulators causes hereditary hemochromatosis, an endocrine disorder of iron overload characterized by chronic hyperabsorption of dietary iron, with deleterious clinical complications if untreated. Epidemiological data suggest that high intake of heme iron, which is abundant in meat products, poses a risk factor for several pathologies, including cardiovascular diseases.
- Iron
- Cardiovascular Disease
1. Nutritional Value of Iron
2. Iron and the Risk for Cardiovascular Disease
References
- Katsarou, A.; Pantopoulos, K. Basics and principles of cellular and systemic iron homeostasis. Mol. Asp. Med. 2020, 75, 100866.
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S.
- Carpenter, C.E.; Mahoney, A.W. Contributions of heme and nonheme iron to human nutrition. Crit. Rev. Food Sci. Nutr. 1992, 31, 333–367.
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843.
- Al-Naseem, A.; Sallam, A.; Choudhury, S.; Thachil, J. Iron deficiency without anaemia: A diagnosis that matters. Clin. Med. 2021, 21, 107–113.
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248.
- De Benoist, B.; Cogswell, M.; Egli, I.; McLean, E. Worldwide Prevalence of Anaemia 1993–2005. In WHO Global Database of Anaemia; WHO: Geneva, Switzerland, 2008.
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259.
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301.
- Pivina, L.; Semenova, Y.; Doşa, M.D.; Dauletyarova, M.; Bjørklund, G. Iron Deficiency, Cognitive Functions, and Neurobehavioral Disorders in Children. J. Mol. Neurosci. 2019, 68, 1–10.
- Pottie, K.; Greenaway, C.; Feightner, J.; Welch, V.; Swinkels, H.; Rashid, M.; Narasiah, L.; Kirmayer, L.J.; Ueffing, E.; MacDonald, N.E.; et al. Evidence-based clinical guidelines for immigrants and refugees. Can. Med. Assoc. J. 2011, 183, E824–E925.
- Khambalia, A.Z.; Aimone, A.M.; Zlotkin, S.H. Burden of anemia among indigenous populations. Nutr. Rev. 2011, 69, 693–719.
- Uauy, R.; Hertrampf, E.; Reddy, M. Iron fortification of foods: Overcoming technical and practical barriers. J. Nutr. 2002, 132, 849S–852S.
- Auerbach, M.; Adamson, J.W. How we diagnose and treat iron deficiency anemia. Am. J. Hematol. 2016, 91, 31–38.
- Weinberg, E.D. The hazards of iron loading. Metallomics 2010, 2, 732–740.
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118535.
- Deugnier, Y.; Turlin, B. Pathology of hepatic iron overload. Semin. Liver Dis. 2011, 31, 260–271.
- Kowdley, K.V. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 2004, 127, S79–S86.
- Utzschneider, K.M.; Kowdley, K.V. Hereditary hemochromatosis and diabetes mellitus: Implications for clinical practice. Nat. Rev. Endocrinol. 2010, 6, 26–33.
- Husar-Memmer, E.; Stadlmayr, A.; Datz, C.; Zwerina, J. HFE-related hemochromatosis: An update for the rheumatologist. Curr. Rheumatol. Rep. 2014, 16, 393.
- Jeney, V. Clinical Impact and Cellular Mechanisms of Iron Overload-Associated Bone Loss. Front. Pharmacol. 2017, 8, 77.
- Kremastinos, D.T.; Farmakis, D. Iron overload cardiomyopathy in clinical practice. Circulation 2011, 124, 2253–2263.
- Pelusi, C.; Gasparini, D.I.; Bianchi, N.; Pasquali, R. Endocrine dysfunction in hereditary hemochromatosis. J. Endocrinol. Investig. 2016, 39, 837–847.
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Pepe, A.; Kattamis, C.; El Kholy, M.; Yassin, M. Diabetes and Glucose Metabolism in Thalassemia Major: An Update. Expert Rev. Hematol. 2016, 9, 401–408.
- Chang, T.P.; Rangan, C. Iron poisoning: A literature-based review of epidemiology, diagnosis, and management. Pediatr. Emerg. Care 2011, 27, 978–985.
- Sullivan, J. Iron and the sex difference in heart disease risk. Lancet 1981, 317, 1293–1294.
- Hunnicutt, J.; He, K.; Xun, P. Dietary iron intake and body iron stores are associated with risk of coronary heart disease in a meta-analysis of prospective cohort studies. J. Nutr. 2014, 144, 359–366.
- Yang, W.; Li, B.; Dong, X.; Zhang, X.Q.; Zeng, Y.; Zhou, J.L.; Tang, Y.H.; Xu, J.J. Is heme iron intake associated with risk of coronary heart disease? A meta-analysis of prospective studies. Eur. J. Nutr. 2014, 53, 395–400.
- Fang, X.; An, P.; Wang, H.; Wang, X.; Shen, X.; Li, X.; Min, J.; Liu, S.; Wang, F. Dietary intake of heme iron and risk of cardiovascular disease: A dose-response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 24–35.
- Han, M.; Guan, L.; Ren, Y.; Zhao, Y.; Liu, D.; Zhang, D.; Liu, L.; Liu, F.; Chen, X.; Cheng, C.; et al. Dietary iron intake and risk of death due to cardiovascular diseases: A systematic review and dose-response meta-analysis of prospective cohort studies. Asia Pac. J. Clin. Nutr. 2020, 29, 309–321.
- Ahluwalia, N.; Genoux, A.; Ferrieres, J.; Perret, B.; Carayol, M.; Drouet, L.; Ruidavets, J.-B. Iron Status Is Associated with Carotid Atherosclerotic Plaques in Middle-Aged Adults. J. Nutr. 2010, 140, 812–816.
- Sawada, H.; Hao, H.; Naito, Y.; Oboshi, M.; Hirotani, S.; Mitsuno, M.; Miyamoto, Y.; Hirota, S.; Masuyama, T. Aortic Iron Overload with Oxidative Stress and Inflammation in Human and Murine Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1507–1514.
- Alnuwaysir, R.I.S.; Hoes, M.F.; van Veldhuisen, D.J.; van der Meer, P.; Grote Beverborg, N. Iron Deficiency in Heart Failure: Mechanisms and Pathophysiology. J. Clin. Med. 2021, 11, 125.
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98.
- Salah, H.M.; Savarese, G.; Rosano, G.M.C.; Ambrosy, A.P.; Mentz, R.J.; Fudim, M. Intravenous iron infusion in patients with heart failure: A systematic review and study-level meta-analysis. ESC Heart Fail. 2023, 10, 1473–1480.
- Araujo, J.A.; Romano, E.L.; Brito, B.E.; Parthé, V.; Romano, M.; Bracho, M.; Montaño, R.F.; Cardier, J. Iron Overload Augments the Development of Atherosclerotic Lesions in Rabbits. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1172–1180.
- Vinchi, F.; Porto, G.; Simmelbauer, A.; Altamura, S.; Passos, S.T.; Garbowski, M.; Silva, A.M.N.; Spaich, S.; Seide, S.E.; Sparla, R.; et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur. Heart J. 2019, 41, 2681–2695.
- Lakhal-Littleton, S.; Wolna, M.; Carr, C.A.; Miller, J.J.J.; Christian, H.C.; Ball, V.; Santos, A.; Diaz, R.; Biggs, D.; Stillion, R.; et al. Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc. Natl. Acad. Sci. USA 2015, 112, 3164–3169.
- Mir, M.B.; Charlebois, E.; Tsyplenkova, S.; Fillebeen, C.; Pantopoulos, K. Cardiac Hamp mRNA Is Predominantly Expressed in the Right Atrium and Does Not Respond to Iron. Int. J. Mol. Sci. 2023, 24, 5163.
- Zhabyeyev, P.; Oudit, G.Y. Unravelling the molecular basis for cardiac iron metabolism and deficiency in heart failure. Eur. Heart J. 2017, 38, 373–375.
- Xu, W.; Barrientos, T.; Mao, L.; Rockman, H.A.; Sauve, A.A.; Andrews, N.C. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep. 2015, 13, 533–545.
- Lakhal-Littleton, S.; Wolna, M.; Chung, Y.J.; Christian, H.C.; Heather, L.C.; Brescia, M.; Ball, V.; Diaz, R.; Santos, A.; Biggs, D.; et al. An essential cell-autonomous role for hepcidin in cardiac iron homeostasis. Elife 2016, 5, e19804.
