Abs1. Intraoduction
Breeding fruit species is time-consuming and expensive. With few exceptions, trees are likely the worst species to work with in terms of genetics and breeding. Most are characterized by large trees, long juvenile periods, and intensive agricultural practice, and environmental variability plays an important role in the heritability evaluations of every single important trait. Although vegetative propagation allows for the production of a significant number of clonal replicates for the evaluation of environmental effects and genotype × environment interactions, the spaces required for plant cultivation and the intensity of work necessary for phenotypic surveys slow down the work of researchers. Fruit breeders are very often interested in fruit traits: size, weight, sugar and acid content, ripening time, fruit storability, and post-harvest practices, among other traits relevant to each individual species. The translation of trait loci and whole-genome sequences into diagnostic genetic markers that are effective and affordable for use by breeders, who must choose genetically superior parents and subsequently choose genetically superior individuals among their progeny, is one of the most difficult tasks still facing tree fruit geneticists. The availability of updated sequencing techniques and powerful software tools offered the opportunity to mine tens of fruit genomes to find out sequence variants potentially useful as molecular markersResearchers devoted to analysing what has been the role of molecular markers in assisting breeders in selection processes, with an emphasis on the fruit traits of the most important fruit crops for which examples of trustworthy molecular markers have been developed, such as the MDo.chr9.4 marker for red skin colour in apples, the CCD4-based marker CPRFC1, and LG3_13.146 marker for flesh colour in peaches, papayas, and cherries, respectively.
12. IntroduMarker-Assisted Selection in Breeding for Fruit
Fruits play an important role in the human diet. Some have sustained and still somehow sustain populations in parts of the world where other food sources are scarcely available. Staple fruits can provide a large part of starch, proteins, and fats from fresh or processed fruits. Staple fruits include some fruit trees that are of paramount importance in some regions of the world. Among others are breadfruit, plantains and bananas, olive oil, and coconut oil. Modern, more evolved societies see fruits and vegetables as sources of beneficial compounds: they are good for our health. They contain natural active principles, ranging from primary metabolites (nutritive factors, vitamins, and minerals) to secondary metabolites known as phytochemicals
[1].
The total world fruit production for 2020 was 887,027,376 metric tons. In 1961, production was 200 million tonnes. The People’s Republic of China, India, and Brazil were found to be the largest producers
[2] (FAOSTAT database). Five fruit species accounted for 57 percent of the total production in 2019, down from 63 percent in 2000: bananas and plantains (18 percent), watermelons (11 percent), apples (10 percent), and oranges and grapes (9 percent each)
[3] (FAOSTAT 2021 Statistical Yearbook).
Undoubtedly, there are several agronomic traits under genetic control that are important for fruit growers and, therefore, for breeders. Researchers decided to collect the information specifically available on the fruit, not because the other characters are of less interest, but because the fruit well represents the final result of a process that starts from the production of the plant in the nursery arriving on the consumer’s table. Having decided that the fuoeview would be the fruit, researchers collected the information object of many years of work of the scientific community that a breeder can have at his disposal. Researchers have collected the information in six sections regarding: (1) phenological traits related to fruit production, (2) skin colour, (3) flesh colour, (4) structural qualities, (5) organoleptic qualities, and (6) harvesting maturation and ripening. The goal was to select the molecular markers available for each species and character and, when not available, indicate the path taken and still to be made to identify markers that are useful and efficient in the selection process.
Consumer choice and consumption behaviour, including healthy perception and the pleasure aspect, given the heterogeneity and the multiplicity of the qualitative aspects that can characterise fruit products, have been widely investigated
[4]. Sensory factors, absence of defects, quality and safety standards, and environmentally friendly products have become a prerequisite for consumers
[4,5,6,7,8][4][5][6][7][8]. Focusing on the topics, consumers’ and marketers’ evaluations of sensory factors, including visual appearance, taste, freshness, colour, aroma, texture, shape, crispness, and absence of fruit defects, have to be considered as a guide for breeders. An example of consumer preference evolution, considering both the appearance and the perception of a higher content of healthy compounds, is the fruit skin and flesh colours: plenty of examples are easily visible in all fruit markets. The deep, intense skin colour selected in all fruit species, as in the apple cultivars ‘Fuji’ and ‘Gala’ clones, as well as the yellow and double yellow/red colour of the kiwifruit cultivars (‘Zespri
®SunGold’, ‘Jingold’, ‘Soreli’, ‘Dorì’, ‘Hongyang’), are just a few examples.
The trait of interest for fruit breeders can be summarised in the following categories: (1) rootstock adaptations to soil pests, diseases, and abiotic stresses; (2) rootstock devoted to minimising the canopy sizes; (3) canopy vigour and adaptation to different training systems; (4) phenology traits (bud break, blooming time, ripening date); (5) pests and disease genetic resistances; (6) fruit size and quality; (7) healthy compounds; (8) fruit resistance to manipulation and post-harvest management.
Considering the fruit, several are the trait of interest: size, shape, weight, skin colour, other skin characteristics (browning, blush), flesh traits (texture, soluble soil content/SSC, sugars, acidity, acids, secondary metabolites), seedlessness, resistance to post-harvest manipulation, and diseases related to the conservation.
Fruit quality traits are indispensable in every fruit breeding program, and selection for other traits has to be considered only from the perspective to add better characteristics in order to get improved cultivars, as well as resistance to pests, for example, avoiding deterioration of quality characteristics. Fruit breeding is a long process due to a usually long juvenility period ranging from one year (strawberry) to decades (avocado, palm date)
[9]. Almost all of today’s commercial products reflect the results of continuing breeding programs, and breeders generally lack the capacity to generate new cultivars quickly in response to evolving consumer preferences and crisis situation, such as sudden pests or disease burst, or climate change. The juvenility period is influenced by the environment
[10] and inversely correlated to vigour
[11]. It is possible to reduce the length of the breeding cycle by manipulating the cultural conditions. In apples, where field-grown seedlings typically do not flower until they are at least 5 years old, plants can be promoted to the adult reproductive phase after as little as 10 months under optimal growth conditions
[12]. In recent years, it has become possible to promote flowering using biotechnologies. Nearly 30 years ago, Detlef Weigel and Ove Nilsson showed that flowering could be triggered in aspen by transgenic expression of a gene from
Arabidopsis called
LEAFY (
LFY)
[13]. From that pioneering work, scientists quickly move to cultivated species, including fruit species such as apple. The overexpression of
BpMADS4 from silver birch (
Betula pendula Roth.)
, a homologous gene of
A. thaliana FRUITFULL which is another key gene in flower initiation, has been found to induce early flowering in apple plants
[14]. By using the
BpMADS4 transgenic apple line T1190 cv. ‘Pinova’, it was shown that one crossbred generation per year is feasible
[15]. These biotechnology tools offer the opportunity to speed up the breeding process that could require many generations of crosses and selections, especially when it is necessary to introduce characters from wild genotypes or cultivars of poor commercial value.
Molecular markers made another advance possible: selecting traits for which the phenotype is not easily scorable until late in seedling cultivation, shortening the breeding cycle and reducing costs. With the explosive growth of genetic research and the wide availability of sequenced genomes as a result of the reduction in sequencing costs achieved through second and third-generation sequencing techniques, a huge number of molecular markers are available. Reference genomes are now accessible in a wide number of fruit species, and information is often collected in public databases which may include collections of multiple economically important species belonging to single botanical families or dedicated to single species (
https://www.rosaceae.org/,
https://kiwifruitgenome.org/,
https://banana-genome-hub.southgreen.fr/,
https://www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/, accessed on 18 May 2023). Although sequencing costs are low and further reductions can be anticipated in the fairly near future, the availability of sequenced genomes is only the first step in the development of truly efficient molecular markers. Molecular markers can be used for several purposes including, phylogeny, molecular fingerprinting, population studies, and assisted selection. In the latter case, it is essential to associate the markers with a trait under simple, Mendelian, or quantitative genetic control. Association mapping or linkage disequilibrium (LD) mapping
[16] or, alternatively, linkage mapping using bi-parental populations
[17,18][17][18] are usually preferred to screen markers located near the trait of interest, although some simplified methods, such as bulk segregant analysis, have been used successfully (
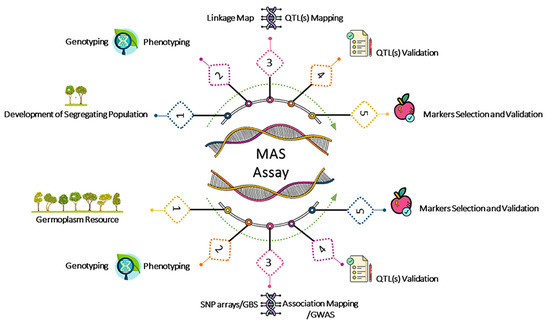
Figure 1)
[19].
Figure 1. Schematic workflow for marker assay development.
Quantitative trait locus (QTL) analysis includes a set of statistical methods that links two types of information: phenotypic data (trait measurements) and genotypic data (usually molecular markers), bridging the gap between genes and the phenotypic traits that result from them
[20]. Considering that most of the traits of agronomic interest are controlled by many genes, QTL analysis is a prerequisite to identifying the molecular markers associated with those traits.
Marker-assisted selection makes it possible to indirectly identify the presence of the genetic determinant or determinants of the traits of interest in the progeny obtained by crossing the appropriate parents. The success of the MAS depends on several factors, including the complexity of the trait considered and the proximity of the marker to the trait to be selected. MAS is gaining considerable importance as it can improve the efficiency of plant breeding through the precise transfer of genomic regions of interest and acceleration of the recovery of the section of the genome that contains the trait of interest.
Genome-wide selection (GWS) allows for the evaluation of the value of parental potentials in a crossing plan. GWS makes use of genomic estimated breeding values (GEBVs) as selection parameters, rather than the estimated breeding values (EBVs) traditionally used by fruit breeders
[21,22][21][22]. GEBV is obtained from the phenotypic analysis of a population that is genotyped with a large number of markers, usually using SNPs, to establish the effect of the markers on complex phenotypes, usually controlled by numerous loci. The GEBV of single individuals is calculated on information obtained from molecular markers in order to select the outstanding individuals. These can be used as parents for further generations of crosses or evaluated as cultivars in extensive field analyses. GWS can be made particularly efficient and cost-effective if MAS is used for screening the cross-population with few markers to eliminate unwanted genotypes. This filtering then allows many thousands of markers to be used in order to apply the GWS
[23]. The foreground MAS selection in a breeding population for simple ‘must-have traits’, such as pest and disease resistances, flesh or skin colour, rootstock dwarfing ability as in apple and cherry, or gender in dioecious crops such as kiwifruit, enable a substantial reduction in the number of seedlings to be genotyped with dense markers for GWS
[24].
It has always been believed that the use of MAS would certainly benefit breeding programs. After all, it seems intuitive that for the characteristics of the fruits, a selection made on the seedlings determines a clear gain in economic terms, considering that a more or less relevant part of the individuals can be discarded. There are not many works that have clearly determined what the genetic and economic gain of the application of MAS could be. A barrier that prevents the diffusion of MAS in breeding programs is the lack of information regarding the cost of applying the technology and the benefits that would derive from it
[25].
When a marker can be regularly used in a breeding program for a certain characteristic without additional testing, it is considered verified. The marker’s alleles must be in substantial linkage disequilibrium with the trait locus’s alleles of interest, and they must be consistently associated with measurable positive or negative effects. These are crucial requirements for validation. Such linkage must be demonstrated in parents of a program’s breeding material.
Alternatively, the markers should be placed close to candidate genes in the genome, which means that functional genomics and fine gene mapping are both beneficial for creating viable markers for MAS.
It can be expected, perhaps not surprisingly, that the use of MAS could be distributed, in terms of effectiveness, in a contrasting way considering two important factors, such as the prediction of breeding value and the reduction of costs. Several studies have demonstrated the superiority of MAS in supporting breeding, particularly in academic studies, where technical resources are frequently available
[26]. Some studies have examined the economic impacts of MAS in horticultural breeding programs. One study indicated that inheritance of the trait, the timing of trait expression, application timing of DNA testing in a program, and testing costs play important roles in determining cost-efficient MAS
[27]. In a breeding program carried out on apple trees, in which the number of seedlings produced each year is very high (30,000), the break-even point (BEP) was evaluated by comparing a traditional selection method with one assisted by molecular markers. The result that emerges clearly shows that an advantage in the use of markers occurred when the rate of removal of seedlings exceeded 13.8%, which constituted the threshold of the BEP
[26]. A multi-trait DNA test for apple acidity, crispness, and firmness on both genetic gain and cost efficiency in an apple seedling population has been confirmed compared to phenotypic selection
[28]. The MASS (marker-assisted seed selection) efficiency calculator is a spreadsheet developed that allows evaluating the efficiency in economic terms and the effectiveness of molecular analyses in the selection, particularly useful in breeding programs of species with long juvenile periods
[29]. The cost reduction threshold has been demonstrated to vary according to the traits considered and the species
[30]. It is to be expected that the economic gain will increase with the decrease in the application costs of molecular markers and with the development of tests that allow the simultaneous evaluation of many traits in the breeding populations.