Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Maria Graça Campos.
Bee pollen (BP)’s richness in nutrients, such as proteins, vitamins, minerals, oligo-elements, and unsaturated fatty acids, is certain, along with the fact that it provides a moderate calorie intake as well as a good level of tolerance and safety, except for in the case of allergic reactions or external pollution, which remain manageable and predictable.
- antioxidant
- dietary
- metabolic
- bee pollen
1. Introduction
Substantial research has been conducted on the composition and pharmacological bioactivities of bee pollen (BP), indicating both its usefulness and safety. Numerous studies have shown that BP has a rich and well-balanced composition to serve as a human food and supplement, while its richness of bioactive compounds, especially polyphenols, confers to it a large array of biological and pharmacological activities [1]. BP-related risks may mainly emanate from external factors [2], or from improper storage and processing conditions [1]. Allergic reactions are rare and BP is perceived as a safe product in most physiological situations including in childhood, in older age, and in disease recoveries [3,4][3][4]. BP from different floral sources has been reported to possess anesthetic, anti-allergic, anti-androgen, anti-atherosclerotic, anti-cancer (anticarcinogenic and anti-mutagenic), anti-inflammatory, antimicrobial (antibacterial, antifungal, and antiviral), antioxidant, antiulcer, and immunostimulant activities [5,6,7,8,9,10,11,12,13][5][6][7][8][9][10][11][12][13]. On metabolic pathophysiology, it has been shown to possess anti-obesity [12], antidiabetic [12], hypocholesterolemic [13], and hepatoprotective [7,12][7][12] effects. In the digestive system, it has been shown to maintain [5], ameliorate [6], and regulate [13] gut functions. In the cardiovascular system, it is able to reduce capillary fragility [5], and can improve overall cardiovascular health [6,13][6][13]. Some authors reported that BP may contribute to the prevention of and positively impact some degenerative processes such as neurodegeneration [6[6][14],14], overall aging [11[11][13],13], and cellular apoptosis [15], and may promote recovery from chronic diseases and possess chemo-preventive properties [7]. It has also been shown to improve skin health and reparation, encompassing many valuable cosmetic qualities [5,16][5][16].
2. Nutritional Value
Bee pollen (BP) hazards can arise mainly from external contamination factors, as pollen is very sensitive to these factors [2], or from inconvenient storage and processing conditions [1]. Special requirements are being established in the ISO-TC34-SC19-WG3 norm to avoid such tampering in the quality assurance and control of the crude material. Allergic reactions to the product can be largely prevented if researchers document pollen composition and preliminarily consider patient sensitivity; BP remains safe for most physiological situations including in childhood, in the elderly and in recovering patients [3,4][3][4]. BP can also be a source of many essential elements for breastfeeding and pregnant women. A recent study of 27 different brand commercial BP samples showed that a daily consumption of 40 g of this product by a breastfeeding woman could bring 23.9%, 22.6%, 23.8%, and 26.3% of daily needs of copper, iron, manganese, and selenium, respectively [33][17]. Similarly, the same study showed that the same schedule consumption by a pregnant woman can supply 31.1%, 30.9%, and 30.7% of copper, manganese, and selenium daily needs, respectively. Moreover, some experimental studies reported that BP had protective effects against prenatal exposure to neurotoxic contaminants in animals [34,35][18][19]. Therefore, it appears a priori to be a very good food in basic nutrition, as a complementary functional food, or also as an adjuvant in different pathophysiological contexts. An abridged diagram of BP composition is presented in Figure 1.
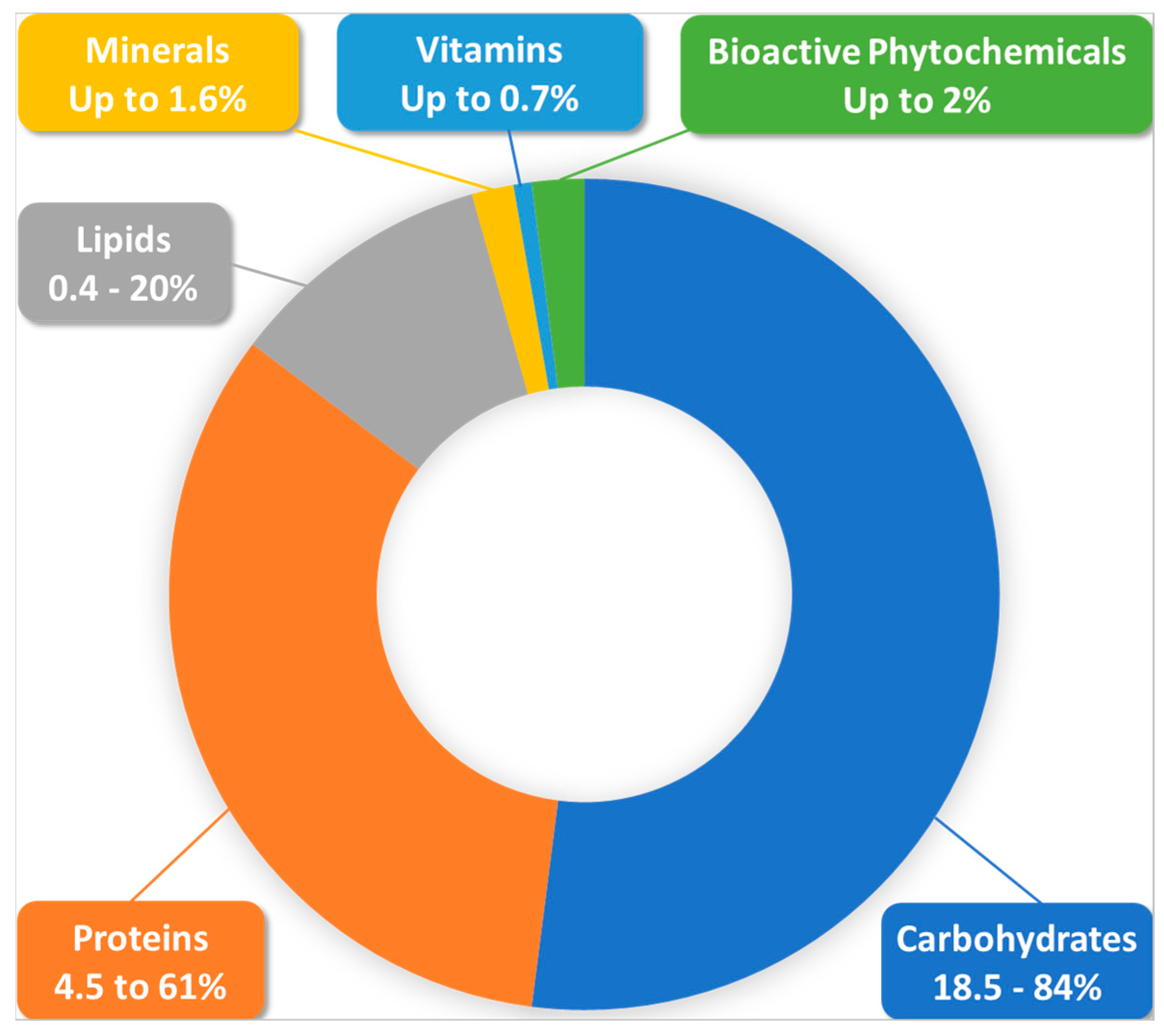
Figure 1.
General Composition of Bee Pollen According to the Currently Available Literature.
According to a recent review of more than 100 published studies, the main components of BP are, in order of weight/weight importance: carbohydrates (percentages from 18.5 to 84% were reported), proteins (4.5–61%), lipids (0.4–20%), fibers, ash, and other components [31,36,37][20][21][22]. Carbohydrates exist here as two types of structurally and functionally distinct pools, namely structural carbohydrates, which contribute to pollen grain structure and protection, and non-structural carbohydrates, which are easily digestible and are generally studied and measured in experimental studies [38][23]. The high carbohydrate content is mainly due to the blending of plant pollen with nectar by honeybees during pellet formation, but is also modulated by plant species, growth level, and harvesting conditions [31][20]. BP carbohydrates consist mainly of monosaccharides with fructose and glucose as the main ingredients, and disaccharides such as sucrose, maltose, maltulose, and trehalose [31,39,40][20][24][25]. Polysaccharides are encountered in pellet-covering layers (e.g., sporopollenin in the exine and cellulose and pectin in the intine) where they play an encapsulating and protective role against physical, biological, and chemical degradation, and do not generally have any known nutritional value [31,39,40][20][24][25].
It Is important, moreover, to note that the abundance of polyols (e.g., mannitol, inositol, xylitol, ribitol), as included components of carbohydrates in this matrix, contribute to its lower caloric value while ensuring the equilibrated import of energy sources and other nutritional needs [5].
The second key components of BP are proteins. Some studies reported that protein content may reach 61% in some pollen types [37][22]. Further to the main botanical origin, the great variation of protein composition may also originate from the fact that BP is mono- or multi-floral [2]. Some comparative analyses reported that pollen was richer in amino acids than eggs, cow meat, and milk [41][26]. The protein content in this product is frequently regarded as a quality index of its nutritional value [7,42][7][27]. Indeed, BP is a natural protein source for honeybees, and thus constitutes a key nutritional supply to ensure the development and growth needs of colony members are met, as well as to serve, via the resulting bee bread [43][28], as a raw material for royal jelly secretion to feed the larvae and queen [44][29]. Protein supplementation from pollen may even have some advantages over other widely used protein supplements such as whey proteins. A study in Wistar rats has reported that this supplementation was more efficient as a hepatoprotective compared to whey protein, both in running and non-running animals. This was manifested particularly by enhanced hepatocyte activity and reduced glycogen deposition [45][30]. Moreover, BP is different from pollen, which is native on the plant. A recent comparative proteomic analysis of bee- and manually-collected pollen of dandelion showed that the bee-collected one contained more metabolism-specific proteins and honeybee proteins which are not found in manually-collected pollen [46][31]. This restudyearch, however, reported that total protein content differed only slightly between the two samples.
Despite its composition variability BP contains all the essential amino acids (AAs) needed by the human body, as well as others used to build proteins in humans [47][32]. However, this does not mean that all types of pollen contain all these AAs. Some floral sources may lack important AAs that are even essential for bee growth and life. Taraxacum officinale pollen, for example, was found to lack tryptophan, phenylalanine, and arginine [46][31]. A sample of Helianthus annuus BP was found to contain phenylalanine at levels that are lower than those needed by honeybees in their normal nutritional requirements [48][33]. Methionine and valine, which are also essential to honeybees, were found to be absent in BP samples from Cyanus segetum and Cytisus scoparius, while present in notable amounts in other BP samples from these same species [49][34]. These differences must be verified by further studies on BP from other species, and those emanating from the natural presence of AAs have to be well distinguished from those originating from experimental procedures. Moreover, in the same geographic location and environmental conditions, BP composition in amino acid could be variable and specific to the individual bee colony [50][35]. Of course, some of them are more widely present than others. A recent study of BP samples from 32 botanical species showed that arginine, asparagine, glutamine, leucine, lysine, and proline constitute about 60% of the total protein content of BP [49][34].
In fact, hundreds of proteins have been isolated in this crude material. One study of four pollen samples, for example, identified 207 proteins in them [3]. To further enhance BP proteinic value, the enzymatic disruption of pollen walls (by an enzymatic mixture of cellulase: pectinase: xylanase: papain, at 4:2:1:3 ratios) was reported to not only enhance protein liberation and availability from inside pollen grains, but also to permit, unlike physical methods (e.g., ultrasound-assisted and/or freeze-thawing wall breaking), the release of the proteins that form these walls [6].
The third main component of BP are lipids [49,52][34][36]. In addition to proteins, lipids appear to play decisive roles in the pollen assembly by honeybees. In fact, it has been shown from different plants and bees species that protein-to-lipid ratios of pollen can drive bee foraging behavior and health [53][37]. This consideration would be crucial if beekeepers want to “guide” bee foraging and naturally adapt the composition of collected BP for human or animal use. Studies in bumblebees have shown that lipid intake is strongly regulated (more than carbohydrates), and that these insects may overeat proteins to reach a sufficient lipid intake or adequate protein–lipid ratio [54][38]. Such behavior is not yet studied in honeybees and may, if verified in social honeybees, give important insights about naturally adapting the composition of BP. Bumblebees and honeybees have been shown to possess several similarities in foraging behaviors, and a similarity in lipid and protein foraging is consequently not impossible. Among behavioral similarities, some floral volatiles were shown to attract both insects [55][39]. A comparative study of honeybee and bumblebee honeys from different geographical and botanical origins reported a similar qualitative composition of free AAs between the two types of honeys, although the quantitative composition was different [56][40]. Another comparative study reported recently that honeybees and bumblebees both avoid foraging nutritionally poor (e.g., having low protein and high lipid content) and highly toxic pollen, compared to other insects [57][41].
BP lipids are mainly composed of phospholipids (mostly glycerophospholipids such as phosphatidylcholines, lysophosphatidylcholines, phosphatidylethanolamines, phosphatidylglycerols, and phosphatidylserines), and polyunsaturated fatty acids, but numerous sphingolipids (mainly ceramides), and glycolipids are also present in BP in smaller quantities [58,59,60][42][43][44]. Sterols are also widely present [54][38], since BP is the only source of these vital molecules for honey bees [61][45]. Phytosterols, viz. sterols, have also been found to drive phylogenetic signals in bee foraging behaviors [49][34] and have been linked to bee colony health and performance [53,54][37][38]. To our current knowledge, stanols in BP, although frequent in plant pollen [62][46] and known for their beneficial effects on human health [63][47], have unfortunately not yet been studied for pharmacological and nutritional purposes.
Long chain fatty acids such as linoleic (Omega-3), α-linolenic (Ω-6), oleic (Ω-9), and α-palmitic acid (saturated) are among the major fatty acids in BP [5,64][5][48]. Unsaturated fatty acids may reach 60% of pollen lipids, while palmitic acid is the most abundant saturated fatty acid [65][49]. Other fatty acids such as oleic acid and myristic acid have also been reported as the main lipidic components [43][28]. The storage conditions [66][50], manipulation and processing of BP may alter its lipidic profile. Drying with different techniques, for example, which is the most common operation in pollen use by humans, was shown to reduce its lipid content and alter the structure of its fatty acids, with freeze-drying having the least impact [59][43]. The lipid profile of BP has also been shown to vary with geographic location and environmental conditions, even for those that are derived from the same plant species [42][27]. Furthermore, harvesting season was also reported to significantly influence lipid profile. An analysis of pollen loads harvested around one year in the same geographic location showed that, not only did the percentage of different fatty acids in the total lipids vary according to the period of collection, but also that the ratio of unsaturated to total fatty acids was variable during the year with a maximum registered in the summer period [66][50].
Pollen lipids are not only important for bee life and in assessing BP nutritional profiles. Recent studies showed that these lipids determine the lipidic content and profile of other derivatives produced by honeybees, namely royal jelly [67][51]. In contrast to proteins, the great impact of the lipidic profile on pollen importance has only just begun to draw attention [68][52].
BP is also rich in many other nutrients in terms of vitamins and minerals, with a content of up to 0.7% of the total weight being constituted by vitamins, and with being a potential source of considerable amounts of fat soluble vitamins (e.g., pro-vitamin A, vitamins D, and E, which is present in many tocopherol derivatives). It could also contain water soluble vitamins (e.g., vitamins of the B group including B1, B2, B3, B5, B6, B7, B9, and B12, and vitamin C) [5,32,40][5][25][53]. BP is, for example, the richest known source of riboflavin in all plant-based materials [69][54]. Interestingly, according to numerous comparative studies from different regions of the world [5], the vitamin content of bee bread appears to be richer than BP (including the presence of vitamin K, which is rarely found in pollen). This may likely result from pollen fermentation by lactic acid bacteria from honeybee stomachs and by the bee bread microbiome [40][25].
Mineral elements are found in BP at an abundant ratio of about 1.6% [33][17]. They include, at largely variable levels between individual taxonomic types, macro-elements such as calcium, magnesium, phosphorus, potassium, and sodium, and microelements such as copper, iron, manganese, selenium, and zinc [5,32,33][5][17][53].
A large number of other micronutrients includes carotenoids [70][55], anthocyanins, glucosinolates, and coenzyme Q10, which are well known for their potential nutritional and health-related effects [5]. Numerous enzymes and coenzymes, as well as nucleic acids, particularly ribonucleic acids, are also universally found in considerable amounts [16,32,71][16][53][56]. Betaines were also recently discovered to be widely present in variable quantities depending on the pollen origin, and have been therefore proposed as additional potential markers for origin identification [72][57]. An analysis was conducted on Spanish BP samples from different botanical origins (e.g., Brassica napus, Vicia sativa, Quercus sp., Retama sp., Papaver sp., Rosa sp., Teucrium sp., Reseda sp., Cytisus sp., Rubus sp.,) and the authors verified the presence of betaines in all of them. Some are pharmacologically well-known molecules, such as betaine, betonicine, trigonelline, and choline, being consistently present at concentration ranges of 7–4910, 264–52,834, 12–3628, and 13–723 mg/kg BP dry weight, respectively [72][57]. These natural and safe molecules, widely known for their protective role against osmotic and oxidative stresses, especially at hepatic and renal levels [73][58], are still very scarcely studied in BP. Their wide presence, if verified by sufficient studies, may give additional support to the bioactivity of BP in human metabolic organs and related physiological functions and pathological processes.
BP also contains an interesting microbiome comprising mainly Lactobacillus strains and other microorganisms such as the Pseudomonas genus and yeasts from Saccharomyces genus [74,75][59][60]. This microbiome evidently originates, at least partly, from the digestive microbiota of the honey bee (bee saliva), and participates in the fermentation of pollen to manufacture bee bread [75][60]. The exploitation of these strains will contribute to increase the value of BP for further nutritional applications, as we will discuss bellow.
A significant observation to underline is that BP pellets in their natural form are hardly digestible and therefore do not allow the full release of their nutrients due to the high protection of the encapsulation walls of the pollen grains. These shells may decrease the bioavailability of micronutrients by as much as 50% or more [4]. Some studies reported that the grinding of pollen grains and their dissolution in warm water may increase nutrient bioavailability from 10–15% (values for raw grains) to 60–80% [76][61]. Studies showed that a preliminary disruption of pollen walls might increase nutrient release and bioavailability [6] and thus enhance the nutritional value and benefits for humans. However, be a “double-edged sword” alter pollen composition, and needs further studies and formulation enhancements.
Fermentation is among the most known processes that may enhance pollen’s nutrient availability. It was reported by many studies that it increases composition richness, either considering nutrients such as vitamins, AAs, peptides, and unsaturated fatty acids, or phytochemicals such as phenolic acids, flavonoids, phenolamides, etc. [69,70,77,78,79,80,81][54][55][62][63][64][65][66]. BP fermentation was found to potentiate bioactivities, such as those that are antioxidant, anti-inflammatory, antibacterial, and antifungal, and to amplify the lowering effects on many metabolic disorder biomarkers, including body mass index, glycemia, cholesterol, and triglyceride levels [70,79,80,81,82][55][64][65][66][67]. These kinds of results were obtained by many well-known and widely used fermenting strains, including bacteria such as Lactococcus lactis, Lactobacillus rhamnosus [82][67], Lactobacillus bulgaricus, and Lactobacillus kunkeei, and yeasts such as Saccharomyces cerevisiae [77][62] and Hanseniaspora uvarum [80][65]. Some studies reported that yeast fermentation resulted in a more pronounced enhancement of nutrient composition than lactic bacteria or mixed (bacteria and yeast strains together) fermentations [83][68]. It has even been reported that fermentation permitted the reduction in allergenicity of BP by significantly reducing its allergen contents and the immunoglobulin-E-binding affinity of these allergens (this was obtained by fermenting Brassica napus BP with Saccharomyces cerevisiae) [84][69]. Indeed, bee bread, which is the natural product of the honeybee-ensured lactic fermentation of BP over the course of approximately one week in the honeycomb, is characterized by a partial alteration of the resistant pollen grain wall and a richer and more balanced nutrient composition, as well as an enhanced digestibility, bio-accessibility, and compound bioavailability in the human body [69,79,82][54][64][67]. The results may evidently vary according to the assessed parameters, but the general tendencies that wresearchers have enumerated here are generally reported by almost all studies.
References
- Campos, M.G.; Frigerio, C.; Bobiş, O.; Urcan, A.C.; Gomes, N.G.M. Infrared irradiation drying impact on bee pollen: Case study on the phenolic composition of Eucalyptus globulus labill and Salix atrocinerea Brot. pollens. Processes 2021, 9, 890.
- Campos, M.G.; Anjos, O.; Chica, M.; Campoy, P.; Nozkova, J.; Almaraz-Abarca, N.; Barreto, L.M.R.C.; Nordi, J.C.; Estevinho, L.M.; Pascoal, A.; et al. Standard methods for pollen research. J. Apic. Res. 2021, 60, 1–109.
- Matuszewska, E.; Plewa, S.; Pietkiewicz, D.; Kossakowski, K.; Matysiak, J.; Rosiński, G.; Matysiak, J. Mass Spectrometry-Based Identification of Bioactive Bee Pollen Proteins: Evaluation of Allergy Risk after Bee Pollen Supplementation. Molecules 2022, 27, 7733.
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Shariati, M.A.; Tešić, L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient—The Present and Perspectives. Biomolecules 2020, 10, 84.
- Baky, M.H.; Abouelela, M.B.; Wang, K.; Farag, M.A. Bee Pollen and Bread as a Super-Food: A Comparative Review of Their Metabolome Composition and Quality Assessment in the Context of Best Recovery Conditions. Molecules 2023, 28, 715.
- Xue, F.; Li, C. Effects of ultrasound assisted cell wall disruption on physicochemical properties of camellia bee pollen protein isolates. Ultrason. Sonochem. 2023, 92, 106249.
- Alshallash, K.S.; Abolaban, G.; Elhamamsy, S.M.; Zaghlool, A.; Nasr, A.; Nagib, A.; El-Hakim, A.F.A.; Zahra, A.A.; Hamdy, A.E.; Taha, I. Bee Pollen as a Functional Product–Chemical Constituents and Nutritional Properties. J. Ecol. Eng. 2023, 24, 173–183.
- Végh, R.; Csóka, M.; Stefanovits-Bányai, É.; Juhász, R.; Sipos, L. Biscuits Enriched with Monofloral Bee Pollens: Nutritional Properties, Techno-Functional Parameters, Sensory Profile, and Consumer Preference. Foods 2022, 12, 18.
- Rojo, S.; Escuredo, O.; Rodríguez-Flores, M.S.; Seijo, M.C. Botanical Origin of Galician Bee Pollen (Northwest Spain) for the Characterization of Phenolic Content and Antioxidant Activity. Foods 2023, 12, 294.
- Hanafy, N.A.N.; Salim, E.I.; Mahfouz, M.E.; Eltonouby, E.A.; Hamed, I.H. Fabrication and characterization of bee pollen extract nanoparticles: Their potential in combination therapy against human A549 lung cancer cells. Food Hydrocoll. Heal. 2023, 3, 100110.
- Chelucci, E.; Chiellini, C.; Cavallero, A.; Gabriele, M. Bio-Functional Activities of Tuscan Bee Pollen. Antioxidants 2023, 12, 115.
- Zhou, E.; Xue, X.; Xu, H.; Zhao, L.; Wu, L.; Li, Q. Effects of covalent conjugation with quercetin and its glycosides on the structure and allergenicity of Bra c p from bee pollen. Food Chem. 2023, 406, 135075.
- Qiao, J.; Feng, Z.; Zhang, Y.; Xiao, X.; Dong, J.; Haubruge, E.; Zhang, H. Phenolamide and flavonoid glycoside profiles of 20 types of monofloral bee pollen. Food Chem. 2023, 405, 134800.
- Oroian, M.; Dranca, F.; Ursachi, F. Characterization of Romanian Bee Pollen—An Important Nutritional Source. Foods 2022, 11, 2633.
- Han, S.; Chen, L.; Zhang, Y.; Xie, S.; Yang, J.; Su, S.; Yao, H.; Shi, P. Lotus Bee Pollen Extract Inhibits Isoproterenol-Induced Hypertrophy via JAK2/STAT3 Signaling Pathway in Rat H9c2 Cells. Antioxidants 2022, 12, 88.
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Al Naggar, Y.; Algethami, A.F.; Shou, Q.; Alsharif, S.M.; et al. Bee Pollen: Clinical Trials and Patent Applications. Nutrients 2022, 14, 2858.
- Sevin, S.; Tutun, H.; Yipel, M.; Aluç, Y.; Ekici, H. Concentration of essential and non-essential elements and carcinogenic/non-carcinogenic health risk assessment of commercial bee pollens from Turkey. J. Trace Elem. Med. Biol. 2023, 75, 127104.
- Ben Bacha, A.; Norah, A.-O.; Al-Osaimi, M.; Harrath, A.H.; Mansour, L.; El-Ansary, A. The therapeutic and protective effects of bee pollen against prenatal methylmercury induced neurotoxicity in rat pups. Metab. Brain Dis. 2020, 35, 215–224.
- Al-Osaimi, M.; El-Ansary, A.; Al-Daihan, S.; Bhat, R.S.; Ben Bacha, A. Therapeutic and Protective Potency of Bee Pollen Against Neurotoxic Effects Induced by Prenatal Exposure of Rats to Methyl Mercury. J. Mol. Neurosci. 2018, 65, 327–335.
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106.
- Crone, M.K.; Grozinger, C.M. Pollen protein and lipid content influence resilience to insecticides in honey bees (Apis mellifera). J. Exp. Biol. 2021, 224, jeb242040.
- Prđun, S.; Svečnjak, L.; Valentić, M.; Marijanović, Z.; Jerković, I. Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles. Foods 2021, 10, 2103.
- Lau, P.; Lesne, P.; Grebenok, R.J.; Rangel, J.; Behmer, T.S. Assessing pollen nutrient content: A unifying approach for the study of bee nutritional ecology. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210510.
- Aylanc, V.; Falcão, S.I.; Vilas-Boas, M. Bee pollen and bee bread nutritional potential: Chemical composition and macronutrient digestibility under in vitro gastrointestinal system. Food Chem. 2023, 413, 135597.
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotics 2020, 9, 811.
- dos Santos, T.R.; Melo, J.d.S.; dos Santos, A.V.; Severino, P.; Lima, S.; Souto, E.B.; Zielińska, A.; Cardoso, J.C. Development of a Protein-Rich By-Product by 23 Factorial Design: Characterization of Its Nutritional Value and Sensory Analysis. Molecules 2022, 27, 8918.
- Hsu, P.-S.; Wu, T.-H.; Huang, M.-Y.; Wang, D.-Y.; Wu, M.-C. Nutritive Value of 11 Bee Pollen Samples from Major Floral Sources in Taiwan. Foods 2021, 10, 2229.
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382.
- Darwish, A.M.G.; El-Wahed, A.A.A.; Shehata, M.G.; El-Seedi, H.R.; Masry, S.H.D.; Khalifa, S.A.M.; Mahfouz, H.M.; El-Sohaimy, S.A. Chemical Profiling and Nutritional Evaluation of Bee Pollen, Bee Bread, and Royal Jelly and Their Role in Functional Fermented Dairy Products. Molecules 2022, 28, 227.
- Jarosz, P.M.; Jasielski, P.P.; Zarobkiewicz, M.K.; Sławiński, M.A.; Wawryk-Gawda, E.; Jodłowska-Jędrych, B. Changes in Histological Structure, Interleukin 12, Smooth Muscle Actin and Nitric Oxide Synthase 1. and 3. Expression in the Liver of Running and Non-Running Wistar Rats Supplemented with Bee Pollen or Whey Protein. Foods 2022, 11, 1131.
- Čeksterytė, V.; Treigytė, G.; Matuzevičius, D.; Jaškūnė, K.; Navakauskas, D.; Kurtinaitienė, B.; Navakauskienė, R. Comparative proteomic profile in bee- and manually-collected Taraxacum officinale pollen. J. Apic. Res. 2022, 61, 543–556.
- Ares, A.M.; Martín, M.T.; Toribio, L.; Bernal, J. Determination of Free Amino Acids in Bee Pollen by Liquid Chromatography with Fluorescence Detection. Food Anal. Methods 2022, 15, 2172–2180.
- Taha, E.-K.A.; Al-Kahtani, S.; Taha, R. Protein content and amino acids composition of bee-pollens from major floral sources in Al-Ahsa, eastern Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 232–237.
- Jeannerod, L.; Carlier, A.; Schatz, B.; Daise, C.; Richel, A.; Agnan, Y.; Baude, M.; Jacquemart, A.-L. Some bee-pollinated plants provide nutritionally incomplete pollen amino acid resources to their pollinators. PLoS ONE 2022, 17, e0269992.
- Sommano, S.R.; Bhat, F.M.; Wongkeaw, M.; Sriwichai, T.; Sunanta, P.; Chuttong, B.; Burgett, M. Amino Acid Profiling and Chemometric Relations of Black Dwarf Honey and Bee Pollen. Front. Nutr. 2020, 7, 558579.
- Chakrabarti, P.; Lucas, H.M.; Sagili, R.R. Evaluating Effects of a Critical Micronutrient (24-Methylenecholesterol) on Honey Bee Physiology. Ann. EÈntomol. Soc. Am. 2020, 113, 176–182.
- Vaudo, A.D.; Tooker, J.F.; Patch, H.M.; Biddinger, D.J.; Coccia, M.; Crone, M.K.; Fiely, M.; Francis, J.S.; Hines, H.M.; Hodges, M.; et al. Pollen Protein: Lipid Macronutrient Ratios May Guide Broad Patterns of Bee Species Floral Preferences. Insects 2020, 11, 132.
- Grund-Mueller, N.; Ruedenauer, F.A.; Spaethe, J.; Leonhardt, S.D. Adding Amino Acids to a Sucrose Diet Is Not Sufficient to Support Longevity of Adult Bumble Bees. Insects 2020, 11, 247.
- Zhang, J.; Liu, J.; Gao, F.; Chen, M.; Jiang, Y.; Zhao, H.; Ma, W. Electrophysiological and Behavioral Responses of Apis mellifera and Bombusterrestris to Melon Flower Volatiles. Insects 2022, 13, 973.
- Dimins, F.; Cinkmanis, I.; Radenkovs, V.; Augspole, I.; Valdovska, A. Analysis of 18 Free Amino Acids in Honeybee and Bumblebee Honey from Eastern and Northern Europe and Central Asia Using HPLC-ESI-TQ-MS/MS Approach Bypassing Derivatization Step. Foods 2022, 11, 2744.
- Hao, K.; Xu, Q.; Huang, S. Pollen-feeding behavior of diverse insects on Geranium delavayi, a flower with large, accessible pollen grains. Am. J. Bot. 2023, 110, e16113.
- Li, Q.; Liang, X.; Xue, X.; Wang, K.; Wu, L. Lipidomics Provides Novel Insights into Understanding the Bee Pollen Lipids Transepithelial Transport and Metabolism in Human Intestinal Cells. J. Agric. Food Chem. 2020, 68, 907–917.
- Wang, J.; Chen, Y.; Zhao, L.; Zhang, Y.; Fang, X. Lipidomics reveals the molecular mechanisms underlying the changes in lipid profiles and lipid oxidation in rape bee pollen dried by different methods. Food Res. Int. 2022, 162, 112104.
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 6833–6848.
- Chakrabarti, P.; Lucas, H.M.; Sagili, R.R. Novel Insights into Dietary Phytosterol Utilization and Its Fate in Honey Bees (Apis mellifera L.). Molecules 2020, 25, 571.
- Zu, P.; Koch, H.; Schwery, O.; Pironon, S.; Phillips, C.; Ondo, I.; Farrell, I.W.; Nes, W.D.; Moore, E.; Wright, G.A.; et al. Pollen sterols are associated with phylogeny and environment but not with pollinator guilds. New Phytol. 2021, 230, 1169–1184.
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322.
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Bin Emran, T.; Simal-Gandara, J. Pharmaceutical Prospects of Bee Products: Special Focus on Anticancer, Antibacterial, Antiviral, and Antiparasitic Properties. Antibiotics 2021, 10, 822.
- Alfawaz, H.A.; El-Ansary, A.; Al-Ayadhi, L.; Bhat, R.S.; Hassan, W.M. Protective Effects of Bee Pollen on Multiple Propionic Acid-Induced Biochemical Autistic Features in a Rat Model. Metabolites 2022, 12, 571.
- Al-Kahtani, S.N.; Taha, E.-K.A.; Farag, S.A.; Taha, R.A.; Abdou, E.A.; Mahfouz, H.M. Harvest Season Significantly Influences the Fatty Acid Composition of Bee Pollen. Biology 2021, 10, 495.
- Zhou, E.; Wang, Q.; Li, X.; Zhu, D.; Niu, Q.; Li, Q.; Wu, L. Effects of Bee Pollen Derived from Acer mono Maxim. or Phellodendron amurense Rupr. on the Lipid Composition of Royal Jelly Secreted by Honeybees. Foods 2023, 12, 625.
- Bennett, M.M.; Welchert, A.C.; Carroll, M.; Shafir, S.; Smith, B.H.; Corby-Harris, V. Unbalanced fatty acid diets impair discrimination ability of honey bee workers to damaged and healthy brood odors. J. Exp. Biol. 2022, 225, jeb244103.
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876.
- Barta, D.G.; Cornea-Cipcigan, M.; Margaoan, R.; Vodnar, D.C. Biotechnological Processes Simulating the Natural Fermentation Process of Bee Bread and Therapeutic Properties—An Overview. Front. Nutr. 2022, 9, 871896.
- Zhang, H.; Zhu, X.; Huang, Q.; Zhang, L.; Liu, X.; Liu, R.; Lu, Q. Antioxidant and anti-inflammatory activities of rape bee pollen after fermentation and their correlation with chemical components by ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry-based untargeted metabolomics. Food Chem. 2023, 409, 135342.
- Miyata, R.; Hoshino, S.; Ahn, M.-R.; Kumazawa, S. Chemical Profiles of Korean Bee Pollens and Their Catechol-O-methyltransferase Inhibitory Activities. J. Agric. Food Chem. 2022, 70, 1174–1181.
- Ares, A.M.; Martín, M.T.; Tapia, J.A.; González-Porto, A.V.; Higes, M.; Martín-Hernández, R.; Bernal, J. Differentiation of bee pollen samples according to the betaines and other quaternary ammonium related compounds content by using a canonical discriminant analysis. Food Res. Int. 2022, 160, 111698.
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456.
- Ilie, C.-I.; Oprea, E.; Geana, E.-I.; Spoiala, A.; Buleandra, M.; Pircalabioru, G.G.; Badea, I.A.; Ficai, D.; Andronescu, E.; Ficai, A.; et al. Bee Pollen Extracts: Chemical Composition, Antioxidant Properties, and Effect on the Growth of Selected Probiotic and Pathogenic Bacteria. Antioxidants 2022, 11, 959.
- Pełka, K.; Otłowska, O.; Worobo, R.W.; Szweda, P. Bee Bread Exhibits Higher Antimicrobial Potential Compared to Bee Pollen. Antibiotics 2021, 10, 125.
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180.
- Zhang, H.; Lu, Q.; Liu, R. Widely targeted metabolomics analysis reveals the effect of fermentation on the chemical composition of bee pollen. Food Chem. 2021, 375, 131908.
- Adaškevičiūtė, V.; Kaškonienė, V.; Barčauskaitė, K.; Kaškonas, P.; Maruška, A. The Impact of Fermentation on Bee Pollen Polyphenolic Compounds Composition. Antioxidants 2022, 11, 645.
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71.
- Filannino, P.; Di Cagno, R.; Vincentini, O.; Pinto, D.; Polo, A.; Maialetti, F.; Porrelli, A.; Gobbetti, M. Nutrients Bioaccessibility and Anti-inflammatory Features of Fermented Bee Pollen: A Comprehensive Investigation. Front. Microbiol. 2021, 12, 622091.
- Yan, S.; Wang, K.; Wang, X.; Ou, A.; Wang, F.; Wu, L.; Xue, X. Effect of fermented bee pollen on metabolic syndrome in high-fat diet-induced mice. Food Sci. Hum. Wellness 2021, 10, 345–355.
- Kaškonienė, V.; Adaškevičiūtė, V.; Kaškonas, P.; Mickienė, R.; Maruška, A. Antimicrobial and antioxidant activities of natural and fermented bee pollen. Food Biosci. 2020, 34, 100532.
- Yan, S.; Li, Q.; Xue, X.; Wang, K.; Zhao, L.; Wu, L. Analysis of improved nutritional composition of bee pollen (Brassica campestris L.) after different fermentation treatments. Int. J. Food Sci. Technol. 2019, 54, 2169–2181.
- Yin, S.; Tao, Y.; Jiang, Y.; Meng, L.; Zhao, L.; Xue, X.; Li, Q.; Wu, L. A Combined Proteomic and Metabolomic Strategy for Allergens Characterization in Natural and Fermented Brassica napus Bee Pollen. Front. Nutr. 2022, 9, 822033.
More
