Dietary iron assimilation is critical for health and essential to prevent iron-deficient states and related comorbidities, such as anemia. The bioavailability of iron is generally low, while its absorption and metabolism are tightly controlled to satisfy metabolic needs and prevent toxicity of excessive iron accumulation. Iron entry into the bloodstream is limited by hepcidin, the iron regulatory hormone. Hepcidin deficiency due to loss-of-function mutations in upstream gene regulators causes hereditary hemochromatosis, an endocrine disorder of iron overload characterized by chronic hyperabsorption of dietary iron, with deleterious clinical complications if untreated. Epidemiological data suggest that high intake of heme iron, which is abundant in meat products, poses a risk factor for several pathologies, including cardiovascular diseases.
1. Nutritional Value of Iron
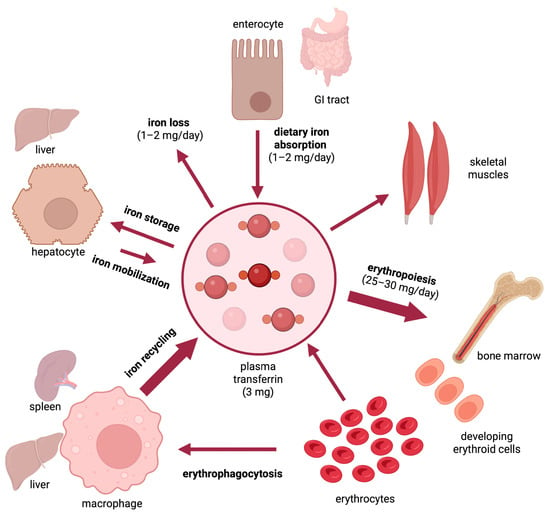
Iron is an essential nutrient that is required for critical biological functions such as oxygen transport and cellular respiration. The adult human body contains 3–5 g of iron, with ~70% utilized in hemoglobin of red blood cells
[1]. The daily iron requirements for erythropoiesis are 25–30 mg and are mostly met by iron recycled from senescent red blood cells, which are cleared by tissue macrophages (
Figure 1). Excess iron is stored in the liver and can be mobilized on demand. As there is no mechanism for iron excretion from the body, dietary absorption of 1–2 mg iron per day is essential to compensate for non-specific iron losses. It should be noted that under physiological conditions, only a small fraction (~15–20%) of ingested luminal iron gets eventually absorbed. This depends on the type of diet, with an estimated 14–18% of iron being absorbed from mixed diets and 5–12% from vegetarian diets
[2]. Body iron stores also affect dietary iron absorption. Thus, in iron-deficient individuals with depleted body iron stores and increased iron demand, the maximum absorption for inorganic iron has been reported at 20% and for heme iron at 35%
[3].
Figure 1.
Dynamics of iron traffic in the human body.
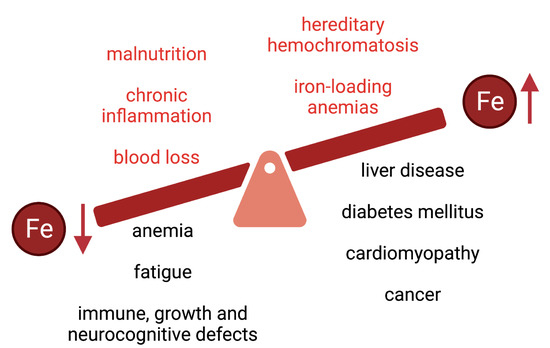
The overall limited bioavailability of iron can lead to iron deficiency anemia or non-anemic iron deficiency, which are the most common pathologies worldwide and remain leading contributors to the global burden of disease (reviewed in
[4][5][6][4,5,6]). Iron deficiency is associated with fatigue and may also lead to immune, growth, and neurocognitive defects (
Figure 2). In 2005, anemias affected roughly a quarter of the world’s population with iron deficiency anemia accounting for about half of these cases
[7]. Little progress has been made since then as, in 2016, iron deficiency anemia was one of the top five causes of years lived with disability with over 1.2 billion cases reported
[8].
Figure 2.
Common causes and clinical complications of iron deficiency and iron overload.
Nutritional guidelines developed by the Food and Nutrition Board at the National Academy of Medicine in the United States recommend that infants between 7–12 months obtain 11 mg of iron from their diet daily, whereas the corresponding values for adult men, menstruating women and pregnant women are 8, 18 and 27 mg of iron, respectively
[9]. These guidelines highlight the elevated iron requirements in pregnant women and infants where iron is critical for growth and development
[10].
Iron deficiency and anemia are more prevalent in vulnerable populations such as indigenous peoples, refugees and immigrants from low and middle-income countries, and disadvantaged subpopulations
[6][11][12][6,11,12]. These notions provide the rationale for food iron fortification programs
[13], and for the use of oral iron supplements or intravenous iron for therapeutic purposes
[14]. Nevertheless, excess body iron may lead to adverse health outcomes
[15], mainly due to the redox reactivity of the metal that can promote oxidative stress and tissue damage
[16]. This is vividly illustrated in diseases of iron overload (
Figure 2) such as hereditary hemochromatosis or iron-loading anemias (including thalassemia), which are associated with type 1 and type 2 diabetes mellitus, arthropathy, osteoporosis, hypogonadism, liver disease (fibrosis, cirrhosis, hepatocellular carcinoma) and cardiomyopathy (reviewed in
[17][18][19][20][21][22][23][24][17,18,19,20,21,22,23,24]). Thus, balanced iron intake is critical to avoid states of iron deficiency or overload.
The hazardous effects of excess iron are also evident in cases of acute iron poisoning, for instance following accidental ingestion of iron supplements by children
[25]. This causes gastric and duodenal mucosal necrosis, the severity of which depends on the amount of iron ingested. Symptoms of iron intoxication include nausea, vomiting, diarrhea, gastrointestinal bleeding, coagulopathy, shock, metabolic acidosis, hepatotoxicity, and abdominal pain.
2. Iron and the Risk for Cardiovascular Disease
The association between iron and cardiovascular disease (CVD) was first proposed in 1981 from observations that the incidence of CVD was elevated in men and post-menopausal women
[26][90]. A meta-analysis of 21 cohort studies with 292,454 participants revealed a significant association between heme iron intake and coronary heart disease incidence
[27][56], with a relative risk of 1.57 (95% CI 1.28 to 1.94). These findings were corroborated in another meta-analysis from 6 different studies including 131,553 participants
[28][57]. Interestingly, total iron intake, serum iron levels, and transferrin saturation were inversely correlated with coronary heart disease incidence
[27][56]. A meta-analysis of 13 primary studies with 252,164 participants reported a relative risk of 1.07 (95% CI 1.01 to 1.14) for CVD in individuals with high dietary heme intake
[29][58]. Another meta-analysis of 19 studies with 720,427 participants reported an association between high dietary heme intake and CVD mortality, with a relative risk of 1.19 (95% CI 1.01 to 1.39)
[30][59]. Iron status has also been positively associated with carotid atherosclerosis in the absence of inflammation
[31][91]. Additionally, abdominal walls from patients having suffered abdominal aortic aneurysms displayed iron accumulation compared to healthy controls with elevated expression of transferrin receptor 1 (TfR1)
[32][92]. On the other hand, iron deficiency is known comorbidity in patients with heart failure
[33][34][93,94] and its correction with intravenous iron administration has been shown to reduce hospitalizations
[35][95]. Taken together, these data highlight epidemiological links between iron status and CVD risk.
Animal studies have provided supportive evidence. Thus, early experiments in rabbits injected with iron dextran and fed a 0.5% cholesterol diet demonstrated greater atherosclerotic lesion development compared to the diet alone
[36][96]. Recent work in mice suggested that non-transferrin-bound iron (NTBI), a highly redox-active form of iron that appears in the circulation primarily during conditions of iron overload, aggravates atherosclerosis
[37][97]. Crossing apolipoprotein E knockout (apoE
–/–) mice, an established model of atherosclerosis, with mice that express a hepcidin-resistant ferroportin mutant (Fpn
wt/C326S) aggravated atherosclerosis via increased levels of NTBI and oxidative stress
[37][97]. Iron loading of the heart appears to be critically important when it is in cardiomyocytes as demonstrated by the reduced survival of mice lacking ferroportin in this cell type
[38][98]. Thus, despite having elevated cardiac iron content, mouse models of hemochromatosis exhibit minor cardiac dysfunction and develop cardiomyopathy only in response to chronic dietary or parenteral iron loading
[39][40][99,100].
The functional importance of cardiomyocyte iron load is also emphasized by the lethal cardiomyopathy documented in mice with iron-deficient cardiomyocytes due to ablation of TfR1
[41][101] or expression of hepcidin-resistant Fpn
C326S [42][102]. Notably, local production of hepcidin is necessary for proper iron homeostasis in the heart
[42][102], and a better understanding of its function and regulation is needed.