Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Marc Bertaux and Version 2 by Alfred Zheng.
Molecular imaging with positron emission tomography is a powerful tool in bladder cancer management. [18F] 2-[18F] fluoro-2-deoxy-D-glucose ([18F]FDG) positron emission tomography (PET)/computed tomography (CT) hass increasingly been used over the years in clinical practice, but the low evidence level of published studies hampers its systematic use being implemented in international guidelines.
- positron emission tomography
- bladder cancer
1. Introduction
Bladder cancer (BC) is the 10th most diagnosed cancer globally, with more than 500,000 new cases and around 200,000 deaths in 2018 [1]. The most important risk factor for developing BC is tobacco smoking, followed by occupational exposure to aromatic amines. In developed countries, more than 90% of BCs are urothelial carcinomas (UC), including a conventional subtype and histomorphologic variants (i.e., microcystic, nested, plasmocytoid etc.), some of which have prognosis implications [2]. Squamous cell carcinomas account for 5% of BC in developed countries, but they are more common in areas where Schistosoma haematobium is endemic. Lastly, 2% of BCs are adenocarcinomas [3]. Patient with BC usually present with painless haematuria. The final diagnosis is made by a histological analysis of tissue resected during transurethral resection of the bladder tumor (TURBT). When it shows non-muscle invasive bladder cancer (NMIBC), the disease can be managed with local treatments and tends to recur but is generally not life-threatening. A systemic imaging work-up for metastatic disease is not needed in that case, except for a few patients with high-risk NMIBC. When muscle-invasive bladder cancer (MIBC) is present (around 30% of patients), i.e., ≥pT2 in the WHO classification [4][5][4,5], a regional and distal cross-sectional imaging work-up must be performed to search for lymph node and distant metastases, as well as concomitant upper urinary tract urothelial carcinoma. According to current guidelines (ESMO, EAU, AUA), it must include a contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen-pelvis combined with a chest CT [6]. To date, [18F] 2-[18F] fluoro-2-deoxy-D-glucose ([18F]FDG) positron emission tomography (PET) is not systematically recommended but is increasingly used in clinical practice. The treatment of MIBC is evolving rapidly.
2. [18F]FDG PET/CT
2.1. Initial Staging and Relapse
2.1.1. Primary Tumor Evaluation
The evaluation of primary tumor in the bladder is mainly performed during cystoscopy. Superficial lesions are directly visible in most cases, and TURBT sample analysis is used to search for bladder muscle invasion. However, TURBT staging performances can vary among urologists. An upstaging from NMBIC to MIBC can happen in 32% of patients when a second TURBT or cystectomy is performed [7][8]. MRI of the bladder can help to specify the depth of neoplastic infiltration in patients. With a dedicated scoring system called VIRADS [8][9], it can accurately help to differentiate MIBC from NMIBC (sensitivity of 90% and specificity of 86% if a score ≥ 3 is considered indicative of MIBC). MRI can also be useful to identify macroscopic peri-vesical fat invasion. On the other hand, [18F]FDG-PET/CT performance for the detection of tumors in the bladder and the upper urinary tract is hampered by the urinary excretion of [18F]FDG. Wang et al. published a meta-analysis regarding the performance of [18F]FDG-PET or PET/CT for detecting bladder lesions and reported a sensitivity of 80.0% (95% CI: 71.0 vs. 87.0%) and a specificity of 84.0% (95% CI: 69.0 vs. 93.0%) [9][10]. To overcome the limitations induced by urinary activity, several studies have proposed adapted protocols to improve [18F]FDG-PET/CT’s performances. These include oral hydration with refilling proposed by Higashiyama et al., which may increase the sensitivity to 92% and specificity to 87% [10][11], and the forced diuresis with furosemide proposed by Nayak et al., which may increase the sensitivity to 96% [11][12]. Some other authors have evaluated the role of early dynamic imaging in [18F]FDG-PET/CT. Yoon et al. compared the sensitivity of early dynamic (10 min after injection), whole-body (60 min after injection), and additional delayed (120 min after injection) PET acquisitions in 52 patients. The sensitivities of early dynamic, whole-body, and additional delayed PET for bladder cancer were 85%, 58%, and 61%, respectively [12][13]. In clinical routine, forced diuresis (20 to 40 mg of furosemide between 0 and 40 min after [18F]FDG injection) with good hydration of the patient is the simplest and most commonly used technique. All in all, [18F]FDG-PET/CT has currently very limited use for T-staging of MIBC (Figure 1).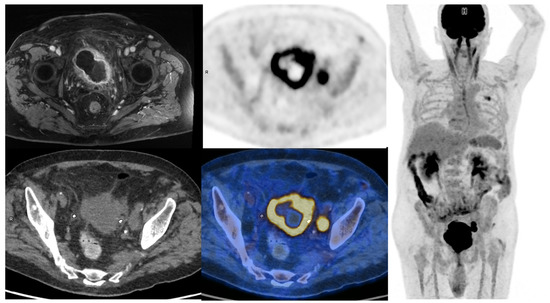
Figure 1. 65-year-old patient with a locally advanced MIBC. Up and left: T1 fat-sat after gadolinium contrast media injection MRI sequence, showing an abnormal enhancement of peri-vesical fat (local stage T3b). Other: PET, CT, and PET/CT fused images revealed a necrotic bladder mass associated with a supra-centimetric ilio-obturator adenopathy on the left side, both with intense FDG uptake. No extra-pelvic lesion was seen (MIP showing focal uptake in the left lung of infectious origin). The patient was managed with first-line chemotherapy, followed by surgery after a good response to treatment.
2.1.2. Regional Nodal Staging
Lymph node involvement is frequent in high-risk MIBC, occurring in approximately 25% of patients with T2 tumors and up to 50% with T3 tumors [13][14][14,15]. Whether in CT or MRI, the current recommendation suggests defining pelvic lymph nodes as suspects for metastasis when they are larger than 8 mm in the small axis [15][16]. This cut-off results in a high false-negative rate [13][14], as many lymph node metastases are smaller, but also in false-positive cases, as some reactive lymph nodes can be significantly enlarged. Studies on the performance of [18F]FDG-PET/CT for N-staging of BC reported sensitivities ranging from 23% to 100%, and specificities ranging from 33% to 100% [16][17][18][17,18,19]. These major discrepancies are probably explained by small sample sizes, differences in population characteristics and acquisition protocols, as well as PET/CT systems and interpretation criteria used. In two recent meta-analyses, there were similar results. Ha et al., in their meta-analysis including 14 studies and 785 patients, found that the pooled sensitivity and specificity of [18F]FDG-PET/CT for initial pelvic lymph node staging were, respectively, 0.57 [95% CI 0.49–0.64] and 0.92 [95% CI 0.87–0.95] in a per-patient analysis [19][20]. Subra et al. found that the pooled sensitivity of [18F]FDG-PET/CT for detecting lymph node metastasis was 0.57 (95% CI 0.29–0.80) and the pooled specificity was 0.95 (95% CI 0.91–1.00) [20][21]. In a prospective study by Girard et al. [18F]FDG-PET/CT outperformed CT in a per-area analysis with diagnostic accuracies of 84% and 78%, respectively. On a per-patient analysis, combining the PET and the CT components of the PET/CT, they found that an additional 8% of patients could be correctly classified compared to CT alone [21][22]. In summary, [18F]FDG-PET/CT seems to slightly improve sensitivity for the detection of pelvic lymph node involvement compared to CT, but in a non-selected population this improvement may be considered too low since both modalities have good specificity but relatively poor sensitivity. [18F]FDG-PET/CT may be particularly useful in selected patients with enlarged lymph nodes seen on morphological imaging, whether to rule out lymph node involvement and enable curative treatment [22][23], or to confirm lymph node neoplastic spread and reveal potential additional metastases (Figure 2 and Figure 3).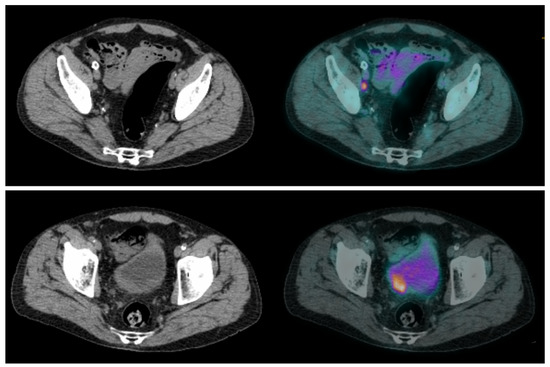
Figure 2. CT and fused FDG-PET/CT images performed at initial staging of a MIBC for a 64-year-old patient. (Bottom line): Intense uptake of the residual primary tumor that was found pT3a at pathological analysis of the cysto-prostatectomy specimen. (Top line): Intense uptake of a single external iliac lymph node of 7 mm of short axis, which did not match criteria to be considered cN+ on CT alone. This lymph node was found pN+ at pathological analysis of the dissection specimen.
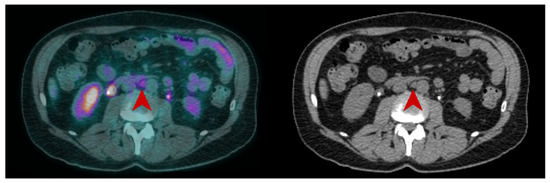
Figure 3. CT and fused FDG-PET/CT images performed at initial staging of a MIBC for a 62year-old man. The patient was classified cN+M0 according to CT alone, and FDG-PET/CT images revealed a moderate uptake of an infra-centimetric lumbar adenopathy (red arrow) that involved restaging to cM1a.
2.1.3. Distant Metastatic Staging
Distant metastases are not rare in patients with MIBC. The most commonly affected sites are extra-pelvic lymph nodes, bone, lungs, liver and peritoneum [23][24][25][24,25,26]. Accurate initial staging is essential for the patients’ management, notably to prevent overtreatment such as futile major surgery, as stated by the EAU-ESMO 2019 consensus [2]. Several studies have evaluated the role of [18F]FDG-PET/CT for the detection of distant metastases, with sensitivities ranging from 54 to 87% and specificities ranging from 90% to 97% in a per-patient analysis [24][25][26][27][25,26,27,28]. In a recent review by Kim, the pooled sensitivity of [18F]FDG PET/CT was superior to that of CT (0.75 vs. 0.43), and with similar specificities (0.95 versus 0.96) [28][29]. [18F]FDG PET/CT could also reveal a second primary cancer that conventional imaging could not [29][30][30,31]. The presence of [18F]FDG-avid extra-pelvic lesions is also an independent predictor of overall survival in MIBC patients [30][31][31,32]. Thus, the benefit of [18F]FDG PET/CT for M-staging of MIBC is well documented, as is its potential impact on patient’s management. In a recent study by Voskuilen et al. including 771 consecutive patients, [18F]FDG PET/CT influenced treatment in 18% of patients. For half of them, it involved a shift from potentially curative to palliative treatment [32][33]. Some ongoing studies such as the phase II EFFORT-MIBC, investigating patients’ management based on [18F]FDG-PET/CT, will provide additional data to consider the implementation of the use of [18F]FDG-PET/CT in current guidelines [33][34]. Currently, the role of [18F]FDG PET/CT in the management of MIBC patients is presented slightly differently across current international guidelines. While the European Association of Urology (EAU) states that its exact role continues to be evaluated, the expert consensus guideline from both the European Society of Medical Oncology (ESMO) and the EAU declares that [18F]FDG-PET/CT should be systematically included in oligometastatic patients staging when considering radical treatment, in order to prevent over treatment [2][6][2,6]. On the other hand, according to American guidelines from the American Urological Association (AUA) and the National Comprehensive Cancer Network (NCCN), [18F]FDG-PET/CT should be used only in selected patients to characterize indeterminate findings found on CT [34][35][35,36] (Figure 4 and Figure 5).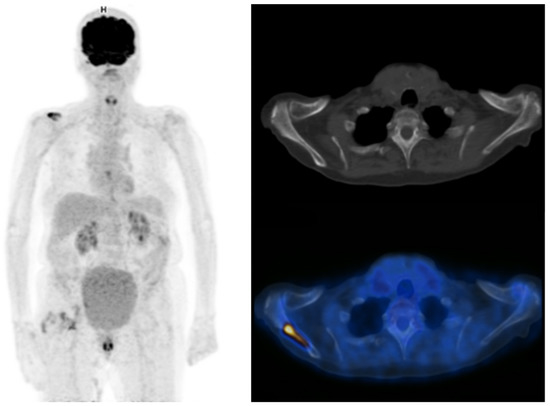
Figure 4. Maximum intensity projection PET, CT and fused FDG-PET/CT images performed at initial staging of a MIBC for an 87-year-old man. The patient was initially classified cN0M0 according to CT alone, and was then upstaged M1b with FDG-PET/CT that highlighted an unknown bone metastasis.
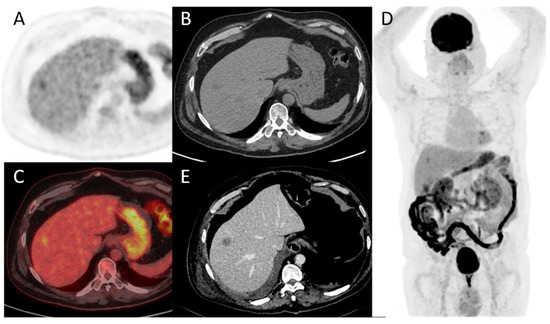
Figure 5. A 78-year-old patient with a diagnosis of urothelial carcinoma. (A–D) PET, CT, fused PET/CT, and PET maximum intensity projection of baseline FDG-PET/CT, respectively. A discreet liver focal liver uptake corresponds to a small hypodensity in unenhanced CT. It was overlooked at the time of diagnosis and the patient underwent a cystectomy with an enterocystoplasty procedure. (E) Follow-up contrast-enhanced CT at 3 months shows an enlargement of the hepatic hypodensity, evocative of its metastatic origin. This case shows that although liver metastases are rare at the time of bladder cancer diagnosis, their detection is paramount.
2.2. Follow-Up
2.2.1. Neoadjuvant and Induction Chemotherapy
Cross-sectional imaging is recommended to evaluate treatment efficiency at the end of both NAC and induction chemotherapy [2]. Although response rates are quite high, with 40–50% of complete pathological response for primary tumors on cystectomy specimens, some patients progress under treatment and should not undergo radical surgery [36][37][37,38]. A few studies have evaluated the performance of [18F]FDG-PET/CT in monitoring the response of BC to preoperative chemotherapy. [18F]FDG-PET/CT identified primary tumor response (partial or complete) with a sensitivity of 75% to 83% and specificity of 80% to 90% [38][39][39,40], and nodal response with a sensitivity of 93% to 100% and a specificity of 17% to 43% [39][40][40,41]. For the detection of overall residual disease, the sensitivity was 75% to 90% and the specificity was 67% to 95% [38][39][41][39,40,42]. Regarding the detection of remaining pelvic LN invasion after preoperative chemotherapy, the sensitivity was 46% to 60% and the specificity was 67% to 71% [30][39][31,40]. Moreover, a complete metabolic response on [18F]FDG-PET/CT was independently associated with a better outcome in terms of cancer-specific survival after a median follow-up of 40 months: hazard ratio of 3.3, IC95% [1.02–10.65] when SUVmax and adapted Deauville criteria were to evaluate the response, and HR 6.32, IC95% [2.06–19.41] when total lesion glycolytic lesion (TLG) measurement was used [41][42]. [18F]FDG-PET/CT provides quite good performance for response monitoring in patients without nodal involvement, and can therefore help to manage neoadjuvant chemotherapy. Nevertheless, a negative [18F]FDG-PET/CT after treatment does not exclude the presence of residual lymph node involvement. Its place in evaluating patients after neoadjuvant chemotherapy needs to be evaluated in a specific and prospective way. To the best of our knowledge, only one study investigated the role of [18F]FDG-PET/CT in evaluating nodal response after preoperative immunotherapy (pembrolizumab). The authors reported that baseline PET/CT could help to select patients with MIBC who are best suited for neoadjuvant immunotherapy strategies. A mediocre diagnostic performance for residual nodal involvement before RC and ePLND (38% sensitivity) was also found in this setting [42][43] (Figure 6).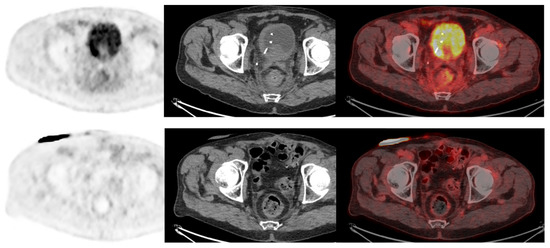
Figure 6. A 73-year-old patient with a diagnosis of urothelial carcinoma. (Upper row): Baseline FDG-PET/CT shows a diffuse pararectal et mesorectal infiltration, with a faint FDG uptake, surgically confirmed as peritoneal metastasis. (Lower row): Same patient seen after 6 cycles of neoadjuvant chemotherapy. FDG-PET/CT shows a complete regression of the posterior pelvis infiltration. The case illustrates the infiltrative nature of low tumor cell density in rare urothelial carcinoma cases, requiring special attention when FDG-PET/CT is used as a systemic staging modality.
