Granulocyte colony-stimulating factor (G-CSF) is a hematopoetic growth factor that is released in response to infection or inflammation to stimulate hematopoetic stem cells to proliferate and generate colonies of differentiated neutrophils. In the therapeutic setting major applications of administration of recombinant G-CSG include: 1) treatment of cyclic and chronic neutropenias; 2) attenuation of the magnitude and duration of chemotherapy-induced neutropenia in cancer patients and 3) mobilization of hematopoetic progenitor cells into peripheral blood to be harvested for stem cell transplantation.
- cancer
- febrile neutropenia
- immunosuppression
- myeloid-derived suppressor cells
- neutrophils
- neutrophil extracellular traps (NETs)
- recombinant granulocyte colony-stimulating factor
- regulatory T cells
- T helper 2 cells
1. Introduction
Colony-stimulating factors (CSFs) are hematopoietic growth factors that are released in response to infection or inflammation to stimulate hematopoietic stem cells to proliferate and generate colonies of differentiated progeny such as neutrophils or macrophages. There are four distinct types of CSFs, each with specific types of receptors, with the occurrence of cross-talk between these receptors, if any, being largely unknown. The CSFs are named after the major types of colonies that they initiate, specifically, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF) and multipotential colony-stimulating factor (most commonly termed interleukin-3) [1].
G-CSF, the focus of the current review, stimulates the proliferation and differentiation of neutrophil precursors, to maintain the number of circulating mature and functional neutrophils.
In the therapeutic setting, major applications of administration of recombinant (r) G-CSF include: (i) attenuation of the magnitude and duration of chemotherapy-induced neutropenia in cancer patients [2], described in greater detail below; (ii) treatment of cyclic and chronic neutropenias [3][4][3,4]; and (iii) mobilization of hematopoietic progenitor cells into peripheral blood to be harvested for stem cell transplantation. In the latter setting, the incidence and severity of experimental acute graft-versus-host disease (aGVHD) are also reduced by administration of rG-CSF [5][6][5,6], seemingly due to the immunoregulatory effects of rG-CSF on cells of both the innate and adaptive immune systems, especially T cells, as reviewed earlier by Yang et al. [7], as well as immature neutrophils [7][8][7,8]. The current review builds on these earlier studies and also addresses the origins and structure of the growth factor and its receptor. Other topics covered include the involvement of tumor-derived G-CSF in tumorigenesis, as well as the therapeutic applications of rG-CSF in medical oncology, in addition to the associated potential risks.
2. Synthesis and Function of G-CSF
Neutrophils are continuously produced from bone marrow-derived pluripotent hematopoietic stem cells via the process of granulopoiesis in which G-CSF plays a key role not only by regulating the production of neutrophils, but also the activity of these cells [9][10][9,10]. G-CSF stimulates the proliferation of hematopoietic progenitors by accelerating their cell cycle rate, thus reducing the transit time through granulopoiesis, via enhancement of the transition of immature metamyelocytes into mature neutrophils and acceleration of their release into the circulation [10]. Apart from mediating the survival of neutrophils and their precursors, G-CSF also promotes the antimicrobial functions of mature neutrophils, such as phagocytosis, superoxide production and pathogen killing [11]. The generation of neutrophils (and other types of granulocytes) from hematopoietic progenitor cells under the influence of various growth factors and cytokines, including G-CSF, is depicted in Figure 1.
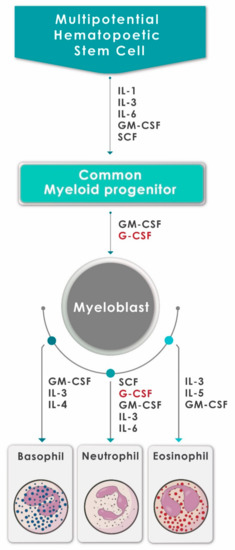
Figure 1. A schematic diagram showing the generation of neutrophils (and other granulocytes) from hematopoietic progenitor cells under the influence of various growth factors and cytokines, including G-CSF (adapted and reproduced from Mehta et al., J Immunol 2015;195:1341–1349; Copyright 2018. The American Association of Immunologists, Inc., Rockville, MD, USA).
Endogenous production of G-CSF is largely triggered by infection and tissue damage in response to production of several pro-inflammatory stimuli such as the cytokines interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNFα), as well as bacterial lipopolysaccharides (LPS) [12][13][12,13]. G-CSF is found in a wide variety of tissue types, but is mainly produced by resting or stimulated stromal cells of the hematopoietic microenvironment (fibroblasts and endothelial cells) and by cells of the innate immune system such as monocytes and macrophages [14].
The importance of G-CSF in the regulation of granulopoiesis became clear following the findings of two early studies based on mice deficient in expression of either G-CSF or the G-CSF receptor (G-CSFR). These mice displayed chronic neutropenia with a corresponding decrease in granulocytic precursors in the bone marrow [15][16][15,16]. Despite these abnormalities, mature neutrophils remained detectable, albeit in much lower numbers, in the blood and bone marrow, indicating the existence of both G-CSF-dependent and -independent mechanisms of granulopoiesis [15][16][15,16], probably involving GM-CSF, IL-3 and IL-6.
According to Toghraie et al. (2019), four different mRNA isoforms resulting from alternative splicing have been reported for G-CSF (transcript variants 1, 2, 3 and 4) [17]. Transcript variants 1 and 2 encode the mature 177-amino acid G-CSF isoform A and the 174-amino acid G-CSF isoform B, respectively [17]. Isoform B is the major isoform produced in prokaryotic and eukaryotic expression systems and has more potent biological activity and greater stability than isoform A [17][18][17,18]. The other recently described G-CSF isoforms are the 141-amino acid isoform C and the 138-amino acid isoform D that are encoded by transcript variants 3 and 4, respectively [17], the activities of which remain to be established.
3. The Role of Endogenous G-CSF in the Pathogenesis of Cancer Progression and Invasion
Various types of advanced, solid malignancies produce G-CSF and also express its receptor, enabling autocrine proliferation of tumor cells, as well as conferring a strategy to intensify the immunosuppressive milieu of the tumor microenvironment (TME) via recruitment of immature and mature neutrophils that function as MDSCs [19][73]. Approximately 10% of patients with advanced, often metastatic, solid malignancies exhibit tumor-related leukocytosis, including, but not limited to, those of the lung, gastrointestinal tract, pancreas, breast and bladder [20][74]. In these settings, tumor-derived G-CSF induces accelerated myelopoiesis that results in a moderate and, in some instances, a profound leukocytosis that is associated with increased numbers of immature myeloid cells. These cells are found in the bone marrow, blood and spleen and their presence is predictive of a poor prognosis [10][19][20][21][22][23][24][25][10,73,74,75,76,77,78,79].
Particularly high levels of expression (~90%) of the G-CSFR have been reported in human gastric and colon cancers, a setting in which G-CSF originates not only from tumor cells per se, but also from stromal myofibroblasts/fibroblasts in the tumor microenvironment (TME) [26][80]. In the case of breast cancer, systemic levels of G-CSF are significantly increased in patients with advanced but not early disease, being highest in those with aggressive, invasive N3 tumors [27][81], with expression of the G-CSFR detected in 71% of patients with invasive ductal adenocarcinomas (stage T1-2 NOMO) [28][82]. In these and other types of advanced breast cancers such as triple negative breast cancer [29][83], as well as the high-risk luminal A dominant breast cancer subtype (C3) [30][84], high-level expression of G-CSF in biopsy specimens is associated with poor overall survival (OS) and invasive potential.
4. Conclusions
Like many other cytokines, G-CSF is a rather enigmatic protein. On the one hand, controlled and appropriate production of G-CSF plays a critical role in anti-infective host defense, while on the other hand, inappropriate production by tumor cells appears to contribute to tumor growth and invasion. In the latter scenario, of which many clinicians may be unaware, the pro-tumorigenic effects are achieved via a dual mechanism of immunosuppression, seemingly by directing T cell polarization towards the Th2 and Treg phenotypes, and, most prominently, via induction of production of MDSCs. Given its key role in host defense, the most effective strategies to neutralize the pro-tumorigenic effects of endogenous G-CSF in the setting of adjunctive anti-cancer therapy are likely to involve pharmacologic targeting of the CXCR1 and CXCR2 chemokine receptors. The involvement of endogenously produced, tumor-derived G-CSF in tumorigenesis contrasts with the beneficial administration of rG-CSF in the prevention of chemotherapy-induced severe and life-threatening infection. Recent innovations in this setting include prophylactic use of rG-CSF, rather than delayed therapeutic administration following the onset of FN, thereby alleviating development of infection and treatment disruption. Although this strategy does not appear to pose the risk of development of hematological and other types of malignancies, ongoing vigilance is necessary, particularly in the context of increasing prophylactic utilization of rG-CSF and the availability of more cost-effective biosimilars. Future studies should be undertaken to monitor the emergence of MDSCs and/or alterations in the levels of their soluble, systemic mediators of immunosuppression in patients with cancer who receive prolonged/frequent administration of rG-CSF.
