You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Jano Varghese and Version 3 by Conner Chen.
Arboviruses consist of a diverse family of pathogens that can infect a wide range of animals and humans. Arboviruses are a diverse family of vector-borne pathogens that include members of the Flaviviridae, Togaviridae, Phenuviridae, Peribunyaviridae, Reoviridae, Asfarviridae, Rhabdoviridae, Orthomyxoviridae and Poxviridae families.
- isothermal amplification
- arboviral diagnostics
- point of care
1. Introduction
Arboviral disease in humans can range from asymptomatic to life-threatening conditions, such as hemorrhagic fevers and encephalitis. Arboviruses are distributed worldwide [1] with some viruses showing restricted geographical distribution (Figure 1). However, as a result of environmental destruction, the travel boom, deforestation, urbanization and failure of vector control programs, arboviruses have expanded into areas not previously seen [2]. Due to the severity and global distribution of arboviral disease, it is vital to have sensitive and rapid diagnostics tests available to determine the causative agent responsible for infection and the implementation of control strategies. Traditionally, serological methods have been the mainstay for the diagnosis of arboviral disease. Methods such as direct and indirect enzyme-linked immunosorbent assays (ELISAs) and lateral flow assays (LFAs) have been widely used to diagnose many arboviral diseases, including Zika, dengue, chikungunya and yellow fever virus. More recently, next-generation sequencing (NGS) approaches have been applied for the detection of arboviruses, including Western bluetongue virus [3], chikungunya, Zika [4], West Nile virus [5] and, in some cases, NGS methods, which have been shown to have a sensitivity similar to that of conventional RT-PCR. Although NGS is well suited for surveillance approaches, these methods are more time consuming and costly, and they require dedicated equipment and computing networks. They are also unsuitable for routine screening when compared to traditional methods. RT-PCR assays tend to be carried out at centralized testing facilities due to the need for thermal cycling systems and related equipment. Advances in molecular biology have led to the discovery of several isothermal nucleic acid amplification technologies (INAATs) that can amplify nucleic acids at a constant temperature without the need for expensive thermal cycling equipment. These newer approaches offer the prospect of decentralizing molecular diagnostic testing and the potential of rapid, cheap point of care (POC) screening and in-field testing applicable to resource-limited settings.
Over the last 150 years, a number of notable epidemics and pandemics have occurred. The influenza A H1N1 pandemic of 1918–1920 resulted in an estimated 50–100 million deaths, the HIV pandemic beginning in the 1980s has affected over 40 million people, and the SARS-CoV-1 epidemic of 1983 and the swine flu pandemic starting in 2009 have caused significant morbidity and mortality. The Ebola outbreaks of 2014–2016 resulted in over 11,000 fatalities, and the Zika outbreaks in 2015–2016 caused a substantial burden of disease globally [6]. Finally, the SARS-CoV-2 pandemic beginning in 2019 has infected nearly 700 million individuals, resulting in nearly 7 million deaths. These outbreaks highlight the importance of novel diagnostics and POC devices that can detect infectious agents in an accurate and timely manner as they arise.
Arboviruses will continue to emerge and re-emerge over time, a notable example being Zika virus which, until recent epidemics, was considered a virus that caused relatively mild infection in humans, but it has since been shown to cause microcephaly and Guillain–Barré syndrome [7]. It has been suggested that one of the more obscure viruses of the Flaviviridae family, such as Spondweni virus (SPOV), Usutu virus (USUV), Ilheus virus (ILHV), Rocio virus (ROCV) and Wesselsbron virus (WSLV), or one of the tick-borne family of flaviviruses could emerge into the human population and cause significant health concerns [7]. Rift Valley fever virus (RVFV) may be one of the next Phleboviruses to emerge as an important human threat due to its continued geographical spread [8]. Alphaviruses such as Mayaro virus (MAYV), which is native to the Americas, may over time adapt to different mosquito populations, such as Aedes, and emerge as a more significant human pathogen [9]. Many arboviruses are found in resource-limited settings that in some cases have inadequate infrastructure for diagnostic testing, emphasizing the importance of inexpensive, rapid and sensitive POC tests that can be used for field deployment.
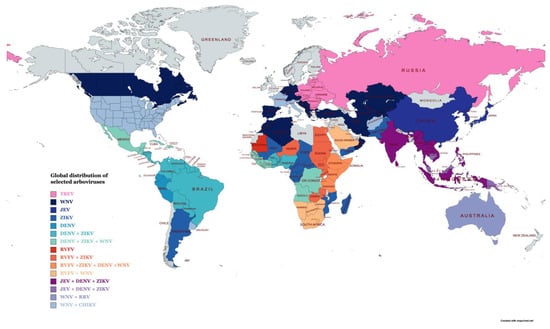
Figure 1. The global distribution of a number of important arboviruses (this map was prepared using information in Socha et al. [1] and references [10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] using the free web-based MapChart software). Table legend abbreviations: tick-borne encephalitis virus (TBEV; West Nile virus (WNV); Japanese encephalitis virus (JEV); Zika Virus (ZIKV); dengue virus (DENV); Rift Valley fever virus (RVFV); Ross River virus (RRV); and chikungunya (CHIKV).
2. Arbovirus Disease in Humans and Animals
Arboviruses consist of a diverse family of pathogens that can infect a wide range of animals and humans. Arboviruses are predominantly positive or negative single-stranded or double-stranded RNA containing viruses from the Flaviviridae, Togaviridae, Phenuviridae, Peribunyaviridae, Reoviridae, Rhabdoviridae, Orthomyxoviridae and Gammaentomopoxvirus families. The only significant DNA-containing virus is the African swine fever virus that belongs to the Asfarviridae family. The 1992 International Catalogue of Arboviruses registered 535 species of virus belonging to 14 families; however, this number is continually increasing due to improvements in isolation and molecular methods for virus discovery and surveillance [34].
Arboviruses must infect their insect vector prior to transmission to a susceptible host [35]. Arboviruses are generally spread as a result of a bite from infected mosquitoes, ticks or other biting flies. Arboviruses circulate among wild animals and birds and are then transmitted as a spill over to humans and domestic animals, which are dead end hosts [36]. Humans and animals infected with arboviruses can display a wide range of symptoms from asymptomatic to life-threatening conditions, such as hemorrhagic fevers and encephalitis, which can often result in long-term complications [8].
A significant number of arboviruses cause human disease and the morbidity and mortality associated with infection cause a substantial social and economic burden when outbreaks occur. Table 1 illustrates a number of arboviruses capable of causing disease in humans. These viruses are distributed on a global scale with some viruses restricted to specific geographical locations corresponding to the distribution of their insect vectors. Mosquitoes are responsible for the transmission of many emerging and re-emerging arboviruses, including the four serogroups of dengue, chikungunya, yellow fever and Zika viruses [37]. These viruses cause a severe burden of disease, with up to 400 million infections and 100 million clinical cases of dengue recorded in 2010 [38].
Table 1.
The table shows a number of important human arboviruses.
| Virus | Family/Order | Vector | Symptoms | Reference |
|---|---|---|---|---|
| Zika virus (ZIKV) |
Flaviviridae | Aedes mosquitoes |
Fever, conjunctivitis, joint pain, headache, maculopapular rash, microcephaly, Guillain–Barré syndrome. |
[10] |
| Yellow fever virus (YFV) |
Flaviviridae | Aedes mosquitoes |
Jaundice, liver damage, gastrointestinal bleeding, recurring fever. |
[11] |
| Dengue Virus (DENV) |
Flaviviridae | Aedes mosquitoes |
Fever, headache, nausea, muscle and joint pain, skin rash, hypovolemic shock, hemorrhage. |
[12] |
| West Nile virus (WNV) |
Flaviviridae | Culex mosquitoes |
Fever, headache, nausea, vomiting, swollen lymph nodes, meningitis, encephalitis, acute flaccid paralysis. |
[13] |
| Japanese encephalitis virus (JEV) |
Flaviviridae | Culex mosquitoes |
Mild flu-like symptoms, encephalitis, seizures, paralysis, coma and long-term brain damage. | [14] |
| Tick-borne encephalitis virus (TBEV) |
Flaviviridae | Ixodes ticks, Dermacentor and Haemaphysalis |
Mild meningitis to severe meningoencephalitis with or without paralysis and long-term brain damage damage. |
[15] |
| Omsk hemorrhagic fever virus (OHFV) |
Flaviviridae | Dermacentor ticks | Fever, headache, nausea, muscle pain, cough and hemorrhages. | [16] |
| Saint Louis encephalitis virus (SLEV) |
Flaviviridae | Culex mosquitoes |
Headache, sensory depression, temporal–spatial disorientation, tremors and changes in consciousness. | [17] |
| Kyasanur Forest disease virus (KFDV) |
Flaviviridae | Haemaphysalis spinigera |
Fever with hemorrhagic and/or neurological features in 20% of patients. | [18] |
| Chikungunya virus (CHIKV) |
Togaviridae | Aedes mosquitoes |
Fever frequently associated with joint pain, polyarthralgia and arthritis, rash, myalgia and headache. | [19] |
| O’nyong nyong virus (ONNV) |
Togaviridae | Anophles mosquitoes |
Low-grade fever, symmetrical polyarthralgia, lymphadenopathy, generalized papular or maculopapular exanthema and joint pain. | [20] |
| Ross river virus (RRV) |
Togaviridae | Culex and Aedes mosquitoes |
Arthritis, rash, fever, fatigue and myalgia. | [21] |
| Eastern equine encephalitis virus (EEEV) |
Togaviridae | Culiseta mosquitoes |
Fever, chills, vomiting, myalgia, arthralgia, malaise and encephalitis. |
[22] |
| Western equine encephalitis virus (WEEV) |
Togaviridae | Aedes, Culex and Culiseta mosquitoes |
Fever, chills, headache, aseptic meningitis and encephalitis. |
[23] |
| Venezuelan equine encephalitis virus (VEEV) |
Togaviridae | Culex mosquitoes |
Fever, chills, malaise, severe headache, myalgia, seizures, drowsiness, confusion and photophobia. | [24] |
| Barmah Forest virus (BFV) |
Togaviridae | Culex and Aedes mosquitoes |
Asymptomatic to relatively mild symptomatic presentations, such as fever and rash; in more severe diseases, polyarthralgia or arthritis. |
[25] |
| Thogoto virus (THOV) |
Orthomyxoviridae | Haemaphysalis and Amblyomma ticks | Benign febrile symptoms to meningoencephalitis. | [26] |
| Rift Valley fever virus (RVFV) |
Bunyvirales | Culex and Aedes mosquitoes |
Fever, headache, backache, vertigo, anorexia, photophobia, hepatitis, jaundice, hemorrhagic disease and ocular complications |
[27][28][27,28] |
| Ngari virus (NRIV) |
Bunyvirales | Aedes, Culex and Anopheles mosquitoes |
Fever, joint pain, rash, can induce severe and fatal hemorrhagic fever | [28] |
| Severe fever with thrombocytopenia syndrome virus (SFTSV) |
Bunyvirales | Haemophysalis Amblyomma, Ixodes and Rhipicephalus ticks |
High fever, gastrointestinal symptoms, thrombocytopenia, leukopenia and multiple organ failure | [29] |
| Crimean–Congo hemorrhagic fever virus (CCHFV) |
Bunyvirales | Hyalomma, Rhipicephalus and Dermacentor ticks |
Non-specific febrile illness, sudden onset of fever, myalgia, diarrhea, nausea and vomiting, hemorrhages at various sites around the body. | [30] |
| Jamestown Canyon virus (JCV) |
Bunyvirales | Aedes, Coquillettidia, Culex mosquitoes |
Non-specific febrile illness, meningitis or meningoencephalitis |
[31] |
| La Crosse encephalitis virus (LACV) |
Bunyvirales | Aedes mosquitoes |
Fever, headache, myalgia, malaise and occasional prostration, encephalitis and lifelong sequelae. | [32] |
| Oropouche Virus (OROV) |
Bunyvirales | Culicoides and Culex mosquitoes |
Acute febrile illness, myalgia, arthralgia, dizziness, photophobia, rash, nausea, vomiting, diarrhea, conjunctive congestion and meningitis. |
[33] |
