Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Domenica Mangieri.
Luteolin (3′,4′,5,7-tetrahydroxyflavone), a member of the flavonoid family derived from plants and fruits, shows a wide range of biomedical applications. In fact, due to its anti-inflammatory, antioxidant and immunomodulatory activities, Asian medicine has been using luteolin for centuries to treat several human diseases, including arthritis, rheumatism, hypertension, neurodegenerative disorders and various infections. Of note, luteolin displays many anti-cancer/anti-metastatic properties.
- phytochemicals
- flavonoids
- metastasis prevention
- signaling pathways
1. Luteolin
Luteolin is a flavonoid belonging to the flavone family; it is isolated from several vegetables and edible herbs, including radicchio, broccoli, raw brussels sprouts, onion leaves, parsley, carrots, peppers and rosemary, where it can occur either as aglycone or bound to one or several carbohydrates as glycoside [67][1] (Figure 21).
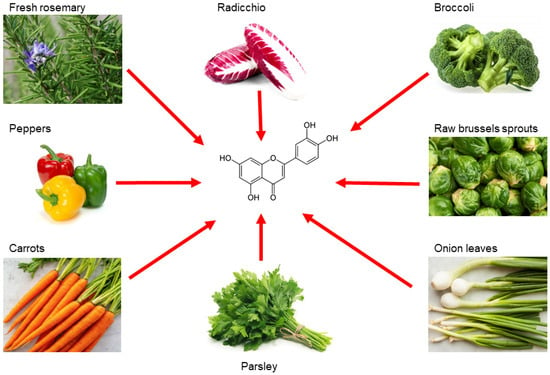
Figure 21. Main sources of luteolin. Edible vegetables, such as radicchio, broccoli, raw brussels sprouts, onion leaves, parsley, carrots, peppers and rosemary are rich in luteolin.
Luteolin is extracted as a yellow crystalline compound and its chemical structure presents a classic flavone C6-C3-C6 skeleton, consisting in two benzene rings with 4 hydroxyl groups located at positions 3, 4, 5 and 7, and one oxygen-containing ring which presents a C2-C3 double bond [67][1]. All these functional groups account for the biological/biochemical properties of luteolin, some of them being specifically involved in maintaining redox balance in various pathological processes [68,69][2][3] (Figure 32).
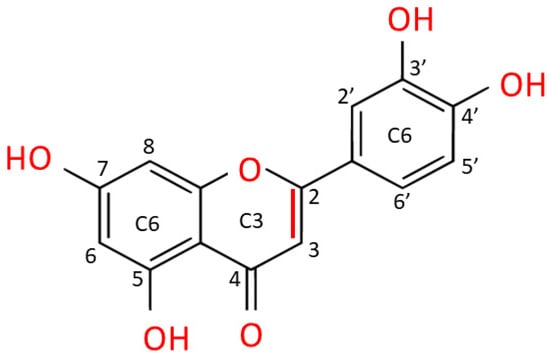
Figure 32. Chemical structure of luteolin (3,4,5,7-tetrahydroxy flavone). Luteolin is a flavone belonging to a group of hydrophobic, naturally occurring compounds, named flavonoids. The functional groups involved in oxido-reductive properties are marked in red.
In fact, preclinical studies ascribed to luteolin several pharmacological properties, including anti-inflammatory, neuroprotective, antimicrobial/antiviral, cardioprotective, antidiabetic and pro-/antioxidant effects [70][4]. Interestingly, since this flavone can interact with various signaling pathways, experimental evidence attributes to luteolin important chemopreventive effects, indicating its ability to interfere with almost all cellular processes underlying cancer development, including metastasis formation [23,24,71][5][6][7]. The available data related to the pharmacokinetics of the free or glycosylated forms of luteolin derive mainly from studies on rat models [72,73,74][8][9][10]. Despite the numerous beneficial effects already mentioned, studies on absorption, metabolism and bioavailability of luteolin in humans are very difficult, partly due to its hydrophobicity, which affects bioavailability and limits the yields of the bioactive flavonoid [70][4].
2. Luteolin Affects the Epithelial-Mesenchymal Transition
EMT is the differentiation process of epithelial cells toward mesenchymal ones; several events are observed during this process, including a disorganization of epithelial cell polarity, the dissolution of cellular junctions, as well as a reorganization of the cytoskeleton [75,76,77][11][12][13]. Thus, EMT involves the downregulation of epithelial markers expression, such as E-cadherin, claudins, zonula occludens-1 (ZO-1), and the acquisition of many facets of the mesenchymal cells, including the expression of N-cadherin and vimentin, coupled with a high propensity to cell motility, invasiveness, and resistance to anoikis [76,77,78][12][13][14]. Several signaling pathways have been identified in EMT induction, such as the transforming growth factor-beta (TGF-β), Notch, Wnt/β-catenin, as well as the Hippo-YAP/TAZ pathways [79,80][15][16]. Moreover, several transcription factors (and their target genes) promote EMT, including Snail1/2, Twist1/2, ZEB1/2 and hypoxia-inducible factors 1/2 (HIF1/2) [81][17]. Physiologically, EMT occurs during embryonic development and in tissue remodeling. In cancer, this process assumes a crucial role in promoting metastasis [76,77][12][13]. Therefore, blocking or reversing EMT may represent an attractive approach to prevent cancer spreading [77][13].
As aforementioned, luteolin inhibits or prevents cell invasion and metastasis in several cancer types, due to a modulation of EMT [82][18]. In this regard, both in vitro and in vivo studies demonstrated that treatment of highly metastatic triple-negative breast cancer (TNBC) with luteolin reduced β-catenin expression and downregulated mesenchymal markers, such as N-cadherin, vimentin, Snail and Slug [25][19]. Moreover, cancer cells regained their epithelial features by overexpressing cell-cell junctional proteins, such as E-cadherin and claudins [25][19].
MicroRNAs (miRs) are small, endogenous, noncoding RNAs that can post-transcriptionally regulate gene expression and play an important role in maintaining normal cellular functions [83][20]. On the other hand, growing evidence shows that some miRs participate in the initiation and progression of cancer, taking part in the EMT process [84,85][21][22]. Luteolin was able to reverse EMT in breast cancer cells (MDA-MB-453 and MCF-7) by overexpressing miR-230, which, in turn, inhibited the Ras/Raf/MEK/ERK signaling pathway, known as a marker of cancer invasiveness [26][23]. Furthermore, luteolin administration significantly inhibited gastric carcinoma (GC) upregulating miR-139, miR-34a, miR-422a, miR-107 levels, while suppressing the oncogenic expression of miR-21, miR-155, miR-224, miR-340 [36][24]. Regulation of this panel of miRs was accompanied by reduced cell proliferation, cell cycle arrest, and induction of apoptosis [36][24]. In a different study, the flavonoid inhibited the oncogenic properties of YAP/TAZ in highly metastatic breast cancer, both in vitro and in xenograft models [27][25]. Moreover, Zang et al. demonstrated that, by interfering with Notch1 and Akt/β-catenin signaling in GC, luteolin reversed the EMT process and, consequently, inhibited tumor progression and invasion, both in vitro and in vivo [35][26]. By using cultures of human lung adenocarcinoma cells (A549), Chen et al. showed that luteolin inhibited cell proliferation and migration through an attenuation of TGF-β1-induced EMT, by activating the PI3K/Akt–NF-κB–Snail signaling pathway [41][27]. Furthermore, luteolin (in a time- and dose-dependent manner) reversed IL-6-induced EMT acting on STAT3 signaling and, consequently, reduced the invasiveness of cancer cells by inhibiting the release of metalloproteases (MMPs) in in vitro models of human pancreatic cancer (i.e., Panc-1 and SW1990 cells) [47][28].
As abovementioned, HIF-1 and HIF-2 are implicated in cancer-associated EMT [81][17]. Based on these premises, Li et al. observed that luteolin, by inhibiting the HIF-1α/VEGF signaling pathway, decreased the expression of N-cadherin and vimentin mesenchymal markers, while augmenting the level of epithelial cadherin isoform in human and murine melanoma cells (i.e., A375 and B16-F10 cell lines, respectively) [50][29]. The inversion of hypoxia-induced EMT was also observed in a murine melanoma model, where luteolin inhibited lung metastasis formation by stopping the β3 integrin/FAK signaling pathway [51][30]. Similarly, luteolin prevented hypoxia-induced EMT of human non-small cell lung carcinoma cells (NSCLC; A549 and NCI-H1975 cell lines), as evidenced by a downregulation of mesenchymal specific markers, such as vimentin and N-cadherin; contextually, luteolin treatment repressed cancer cell motility and adhesion, interfering with integrin β1 expression and with the FAK-signaling pathway [42][31].
The ability of luteolin to reverse EMT in cancer cells is often enhanced by adding other flavonoids, such as quercetin [86,87][32][33]. Indeed, a mixture of luteolin and quercetin was able to attenuate (in a time- and dose-dependent manner) EMT-correlated events, migration and invasiveness of human squamous carcinoma, both in vivo and in vitro, by suppressing the Src/Stat3/S100A signaling pathway [86][32]. Similarly, the addition of luteolin to quercetin inhibited metastasis of skin squamous carcinoma by blocking the Akt/mTOR/c-Myc signaling pathway to suppress RPS19-activated EMT signaling [87][33].
In summary, luteolin appears capable to block or reverse EMT by acting on multiple molecular targets, hindering the first steps of cancer spreading (Figure 43).
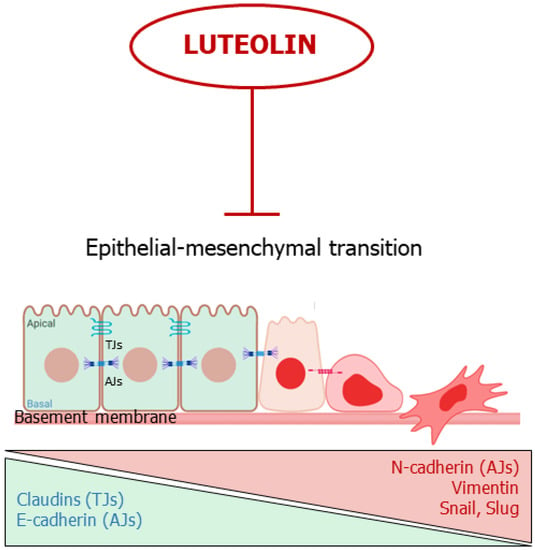
Figure 43.
Luteolin interferes with the epithelial-mesenchymal transition. The flavone inhibits or reverses EMT. TJs: Tights Junctions; AJs; Adherens Junctions.
3. Luteolin Suppresses Angiogenesis
Angiogenesis is the process by which new blood vessels form, starting from preexisting vasculature; this event depends on pro-angiogenic mediators, such as vascular endothelial growth factor (VEGF), basic-fibroblast growth factor (bFGF), metalloproteases, etc., and on negative regulators of angiogenesis, including thrombospondin and endostatin [88][34]. Angiogenesis plays a crucial role during a variety of physiological processes, such as wound healing, embryonic development and pregnancy [88][34]. On the other hand, neovascularization is a key event for pathological processes, such as tumor progression, invasion and metastatic cascade [89][35]. This last aspect is pharmacologically relevant: in fact, many authorized cancer therapies are directed against the tumor-associated vessels [89,90,91,92][35][36][37][38].
Several investigations demonstrate that various flavonoids—including luteolin—act as negative regulators of VEGF and other pro-angiogenic factors [93,94,95][39][40][41]. For instance, Cai and co-workers demonstrated that luteolin decreased VEGF secretion and VEGF mRNA expression in pancreatic carcinoma cells (PANC-1, CoLo-357 and BxPC-3 cell lines), via inhibition of the NF-κB transcriptional factor activity [48][42]. Furthermore, treating human choroidal melanoma cells (C918 and OCM-1) with luteolin not only inhibited VEGF expression, but also increased cancer cell death [54][43]. Additionally, an in vitro study on breast cancer showed that luteolin, by interfering with Notch1 expression, inhibited VEGF secretion from tumor cells, decreased endothelial cell migration, proliferation, and their propension to form tube-like structures on a Matrigel layer [37][44]. Cook and co-coworkers demonstrated the ability of luteolin to block the production of VEGF in human breast cancer cells (T47-D and BT-474) responsive to (natural and synthetic) progestins, both in vitro and in a xenograft model [28][45]. In a similar study, the authors showed that luteolin (in a dose- and time-dependent fashion) significantly reduced VEGF secretion in human TNBC cells (i.e., MDA- MB-435 and highly aggressive MDA-MB-231 (4175) LM2), coupled with a significantly decreased cell viability and reduced migration and invasion in vitro; at the same time, the authors showed that the flavonoid inhibited lung metastasis formation in a dedicated xenograft model [29][46].
The anti-proliferative/anti-mitotic effect on endothelial cells was also tested in infantile haemangioma in vitro and in vivo, by using haemangioma-derived stem cells (HemSC) [58][47]. In detail, the results of this study demonstrated that luteolin suppressed VEGF expression and inhibited HemSC growth in a dose-dependent manner, and, at the same time, the flavonoid inhibited both angiogenesis and vasculogenesis, in a murine model, by acting on the frizzled6 (FZD6) signaling pathway [58][47]. In prostate cancer, luteolin strongly suppressed neovascularization in different experimental settings by inhibiting the activation of VEGFR-2/AKT/ERK/mTOR/P70S6K signaling pathways [56][48]. In fact, it abolished angiogenesis in an ex vivo chicken chorioallantoic membrane (CAM) assay and in a Matrigel plug assay; in addition, the flavone suppressed both vascularization and growth of the tumor in a xenograft model [56][48]. Moreover, luteolin inhibited different pathways of vascularization in an in vitro model of uveal melanoma, including angiogenesis, vasculogenesis and vasculogenic mimicry, by suppressing the PI3K/AKT signaling pathway [52][49].
As previously cited, miRs have a role in cancer evolution, including angiogenesis [85][22]. In an experimental model of NSCLC, luteolin repressed the expression of the rich element binding protein B (PURB) and crucial proangiogenic factors, including VEGF and MMP-2/-9, by overexpressing the miR-133a-p69 and by regulating MAPK and PI3K/Akt signaling pathways [43][50]. In breast cancer, the flavonoid upregulated the biogenesis of miR-34a, miR-181a, miR-139-5p, miR-224 and miR-246, while it decreased the level of miR-155, coupled with a significant inhibition of VEGF/Notch signaling and MMPs downregulation [30][51].
Tumor growth and its progression also depend on proangiogenic factors secreted by the surrounding microenvironment cells (e.g., fibroblasts, tumor associated macrophages (TAMs), etc.) [8][52]. Fang et al. demonstrated that luteolin inhibited the ability of TAMs to induce angiogenesis, thereby inhibiting tumor growth and its spreading, in both normoxic and hypoxic conditions [96][53].
Taken together, these studies show promise for luteolin as a potent anti-angiogenic agent, evading tumor evolution and metastatic cascade (Figure 54).
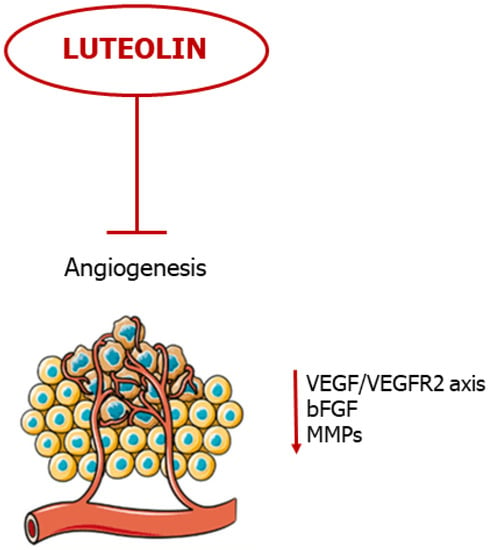
Figure 54.
Luteolin blocks neovascularization. The flavonoid blocks angiogenesis by negative regulation of pro-angiogenic mediators.
References
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59.
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34.
- Xu, H.; Linn, B.; Zhang, Y.; Ren, J. A review on the antioxidative and prooxidative properties of luteolin. React. Oxyg. Species 2019, 7, 136–147.
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974.
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612.
- Tuli, H.S.; Rath, P.; Chauhan, A.; Sak, K.; Aggarwal, D.; Choudhary, R.; Sharma, U.; Vashishth, K.; Sharma, S.; Kumar, M.; et al. Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives. Cancers 2022, 14, 5373.
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Zacconi, F.; De Rubis, G.; Gupta, G.; Sharifi-Rad, J.; Cho, W.C.; et al. Luteolin: A flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 2022, 22, 386.
- Zhou, P.; Li, L.P.; Luo, S.Q.; Jiang, H.D.; Zeng, S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J. Agric. Food Chem. 2008, 56, 296–300.
- Deng, C.; Gao, C.; Tian, X. Pharmacokinetics, tissue distribution and excretion of luteolin and its major metabolites in rats: Metabolites predominate in blood, tissues and are mainly excreted via bile. J. Funct. Foods. 2017, 35, 332–340.
- Hayasaka, N.; Shimizu, N.; Komoda, T.; Mohri, S.; Tsushida, T.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Absorption and Metabolism of Luteolin in Rats and Humans in Relation to in Vitro Anti-inflammatory Effects. J. Agric. Food Chem. 2018, 66, 11320–11329.
- Derynck, R.; Muthusamy, B.P.; Saeteurn, K.Y. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2014, 31, 56–66.
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal 2014, 7, re8.
- Singh, M.; Yelle, N.; Venugopal, C.; Singh, S.K. EMT: Mechanisms and therapeutic implications. Pharmacol. Ther. 2018, 182, 80–94.
- Scheel, C.; Onder, T.; Karnoub, A.; Weinberg, R.A. Adaptation versus selection: The origins of metastatic behavior. Cancer Res. 2007, 67, 11476–11480.
- Deshmukh, A.P.; Vasaikar, S.V.; Tomczak, K.; Tripathi, S.; den Hollander, P.; Arslan, E.; Chakraborty, P.; Soundararajan, R.; Jolly, M.K.; Rai, K.; et al. Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc. Natl. Acad. Sci. USA 2021, 118, e2102050118.
- Li, Z.; Wang, Y.; Zhu, Y.; Yuan, C.; Wang, D.; Zhang, W.; Qi, B.; Qiu, J.; Song, X.; Ye, J.; et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol. Oncol. 2015, 9, 1091–1105.
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196.
- Hussain, Y.; Cui, J.H.; Khan, H.; Aschner, M.; Batiha, G.E.; Jeandet, P. Luteolin and cancer metastasis suppression: Focus on the role of epithelial to mesenchymal transition. Med. Oncol. 2021, 38, 66.
- Lin, D.; Kuang, G.; Wan, J.; Zhang, X.; Li, H.; Gong, X.; Li, H. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 2017, 37, 895–902.
- Kim, V.N. Small RNAs: Classification, biogenesis, and function. Mol. Cells 2005, 19, 1–15.
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. (Lond.) 2021, 41, 199–217.
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–227.
- Gao, G.; Ge, R.; Li, Y.; Liu, S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3265–3271.
- Pu, Y.; Zhang, T.; Wang, J.; Mao, Z.; Duan, B.; Long, Y.; Xue, F.; Liu, D.; Liu, S.G.Z. Luteolin exerts an anticancer effect on gastric cancer cells through multiple signaling pathways and regulating miRNAs. J. Cancer 2018, 9, 3669–3675.
- Cao, D.; Zhu, G.Y.; Lu, Y.; Yang, A.; Chen, D.; Huang, H.J.; Peng, S.X.; Chen, L.W.; Li, Y.W. Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed. Pharmacother. 2020, 129, 110462.
- Zang, M.D.; Hu, L.; Fan, Z.Y.; Wang, H.X.; Zhu, Z.L.; Cao, S.; Wu, X.Y.; Li, J.F.; Su, L.P.; Li, C.; et al. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J. Transl. Med. 2017, 15, 52.
- Chen, K.C.; Chen, C.Y.; Lin, C.R.; Yang, T.Y.; Chen, T.H.; Wu, L.C.; Wu, C.C. Luteolin attenuates TGF-β1-induced epithelial-mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt-NF-κB-Snail pathway. Life Sci. 2013, 93, 924–933.
- Huang, X.; Dai, S.; Dai, J.; Xiao, Y.; Bai, Y.; Chen, B.; Zhou, M. Luteolin decreases invasiveness, deactivates STAT3 signaling, and reverses interleukin-6 induced epithelial-mesenchymal transition and matrix metalloproteinase secretion of pancreatic cancer cells. OncoTargets Ther. 2015, 8, 2989–3001.
- Li, C.; Wang, Q.; Shen, S.; Wei, X.; Li, G. HIF-1α/VEGF signaling-mediated epithelial-mesenchymal transition and angiogenesis is critically involved in anti-metastasis effect of luteolin in melanoma cells. Phyther. Res. 2019, 33, 798–807.
- Ruan, J.S.; Liu, Y.P.; Zhang, L.; Yan, L.G.; Fan, F.T.; Shen, C.S.; Wang, A.Y.; Zheng, S.Z.; Wang, S.M.; Lu, Y. Luteolin reduces the invasive potential of malignant melanoma cells by targeting β3 integrin and the epithelial-mesenchymal transition. Acta Pharmacol. Sin. 2012, 33, 1325–1331.
- Ruan, J.; Zhang, L.; Yan, L.; Liu, Y.; Yue, Z.; Chen, L.; Wang, A.Y.; Chen, W.; Zheng, S.; Wang, S.; et al. Inhibition of hypoxia-induced epithelial mesenchymal transition by luteolin in non-small cell lung cancer cells. Mol. Med. Rep. 2012, 6, 232–238.
- Fan, J.J.; Hsu, W.H.; Lee, K.H.; Chen, K.C.; Lin, C.W.; Lee, Y.A.; Ko, T.P.; Lee, L.T.; Lee, M.T.; Chang, M.S.; et al. Dietary Flavonoids Luteolin and Quercetin Inhibit Migration and Invasion of Squamous Carcinoma through Reduction of Src/Stat3/S100A7 Signaling. Antioxidants 2019, 8, 557.
- Chen, K.C.; Hsu, W.H.; Ho, J.Y.; Lin, C.W.; Chu, C.Y.; Kandaswami, C.C.; Lee, M.T.; Cheng, C.H. Flavonoids Luteolin and Quercetin Inhibit RPS19 and contributes to metastasis of cancer cells through c-Myc reduction. J. Food Drug Anal. 2018, 26, 1180–1191.
- Liekens, S.; De Clercq, E.; Neyts, J. Angiogenesis: Regulators and clinical applications. Biochem. Pharmacol. 2001, 61, 253–270.
- Carmeliet, P.J.R. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307.
- Niu, G.; Chen, X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 2010, 11, 1000–1017.
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015, 35, S224–S243.
- Mukherjee, A.; Madamsetty, V.S.; Paul, M.K.; Mukherjee, S. Recent Advancements of Nanomedicine towards Antiangiogenic Therapy in Cancer. Int. J. Mol. Sci. 2020, 21, 455.
- Martínez-Poveda, B.; Torres-Vargas, J.A.; Ocaña, M.D.C.; García-Caballero, M.; Medina, M.Á.; Quesada, A.R. The Mediterranean Diet, a Rich Source of Angiopreventive Compounds in Cancer. Nutrients 2019, 11, 2036.
- Tosetti, F.; Ferrari, N.; De Flora, S.; Albini, A. Angioprevention’: Angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002, 16, 2–14.
- Bagli, E.; Stefaniotou, M.; Morbidelli, L.; Ziche, M.; Psillas, K.; Murphy, C.; Fotsis, T. Luteolin inhibits vascular endothelial growth factor-induced angiogenesis; inhibition of endothelial cell survival and proliferation by targeting phosphatidylinositol 3’-kinase activity. Cancer Res. 2004, 64, 7936–7946.
- Cai, X.; Lu, W.; Ye, T.; Lu, M.; Wang, J.; Huo, J.; Qian, S.; Wang, X.; Cao, P. The molecular mechanism of luteolin-induced apoptosis is potentially related to inhibition of angiogenesis in human pancreatic carcinoma cells. Oncol. Rep. 2012, 28, 1353–1361.
- Shi, M.L.; Chen, Y.F.; Liao, H.F. Effect of luteolin on apoptosis and vascular endothelial growth factor in human choroidal melanoma cells. Int. J. Ophthalmol. 2021, 14, 186–193.
- Zang, M.; Hu, L.; Zhang, B.; Zhu, Z.; Li, J.; Zhu, Z.; Yan, M.; Liu, B. Luteolin suppresses angiogenesis and vasculogenic mimicry formation through inhibiting Notch1-VEGF signaling in gastric cancer. Biochem. Biophys. Res. Commun. 2017, 490, 913–919.
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Goyette, S.; Mafuvadze, B.; Hyder, S.M. Luteolin inhibits progestin-dependent angiogenesis, stem cell-like characteristics, and growth of human breast cancer xenografts. Springerplus 2015, 4, 444.
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Hyder, S.M. Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells. Breast Cancer Dove Med. Press 2016, 9, 9–19.
- Dai, Y.; Zheng, H.; Liu, Z.; Wang, Y.; Hu, W. The flavonoid luteolin suppresses infantile hemangioma by targeting FZD6 in the Wnt pathway. Investig. New Drugs 2021, 39, 775–784.
- Pratheeshkumar, P.; Son, Y.O.; Budhraja, A.; Wang, X.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.C.; Kim, D.; Divya, S.P.; et al. Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. PLoS ONE 2012, 7, e52279.
- Chen, Y.F.; Wu, S.; Li, X.; Chen, M.; Liao, H.F. Luteolin Suppresses Three Angiogenesis Modes and Cell Interaction in Uveal Melanoma in Vitro. Curr. Eye Res. 2022, 47, 1590–1599.
- Pan, J.; Cai, X.; Zheng, X.; Zhu, X.; Feng, J.; Wang, X. Luteolin inhibits viability, migration, angiogenesis and invasion of non-small cell lung cancer vascular endothelial cells via miR-133a-3p/purine rich element binding protein B-mediated MAPK and PI3K/Akt signaling pathways. Tissue Cell 2022, 75, 101740.
- Sun, D.W.; Zhang, H.D.; Mao, L.; Mao, C.F.; Chen, W.; Cui, M.; Ma, R.; Cao, H.X.; Jing, C.W.; Wang, Z.; et al. Luteolin Inhibits Breast Cancer Development and Progression In Vitro and In Vivo by Suppressing Notch Signaling and Regulating MiRNAs. Cell Physiol. Biochem. 2015, 37, 1693–1711.
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70.
- Fang, B.; Chen, X.; Wu, M.; Kong, H.; Chu, G.; Zhou, Z.; Zhang, C.; Chen, B. Luteolin inhibits angiogenesis of the M2-like TAMs via the downregulation of hypoxia inducible factor-1α and the STAT3 signalling pathway under hypoxia. Mol. Med. Rep. 2018, 18, 2914–2922.
More
