Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Sonia Morè.
Belantamab Mafodotin, a first-in-class anti-B cell maturation antigen (BCMA) antibody–drug conjugate, demonstrated good efficacy and safety profile in triple-refractory patients in the phase 2 DREAMM-2 trial, and it was approved for the treatment of MM triple-exposed patients with >4 prior lines of therapy.
- multiple myeloma
- belantamab mafodotin
- antibody–drug conjugate
1. Introduction
Multiple myeloma (MM) is the second most common hematological disease accounting for approximately 10% of all hematological malignancies, and its typically an elderly hematologic disease, with the median age of patients at the diagnosis of about 65 years [1].
MM is characterized by abnormal plasma cell proliferation in the bone marrow, which produces an excess of monoclonal protein (M-protein) detected in blood and urine. This M-protein causes specific organ damage resulting in MM signs and symptoms, typically hypercalcemia, renal insufficiency, anemia and osteolytic bone lesions [2].
The therapeutical landscape of effective anti-myeloma drugs has widely expanded in the last decade thanks to the introduction of proteasome inhibitors (PIs, such as bortezomib, carfilzomib and ixazomib), immunomodulatory drugs (IMiDs, such as thalidomide, lenalidomide and pomalidomide) and, recently, monoclonal antibodies (mAbs), such as elotuzumab, daratumumab and isatuximab [2]. The introduction of these agents has translated into prolonged progression-free survival (PFS) as well as overall survival (OS) with significantly fewer toxicities and improved quality of life. Currently, thanks to the therapeutic first-line approaches, including quadruplet induction combination, high-dose chemotherapy, followed by autologous hematopoietic stem-cells transplantation (ASCT), and consolidation–maintenance, the 10-year OS probability is about 60% in patients eligible for ASCT. [3]. In non-transplant eligible (NTE) patients, therapeutical first-line approaches based on doublet or triplet combination with PIs, IMiDs and mAbs result in a prolonged OS, with a median OS of 5 years.
However, MM still remains largely an incurable disease with poor outcomes, especially among patients who become resistant to therapies [4].
Immunotherapeutic approaches, both passive, for example, mAbs and cellular products targeting clonal plasma cells, or active (when a patient’s immune system is stimulated to induce an immune response against plasma cells), have been investigated to harness the patients’ immune system to destroy clonal plasma cells and, nowadays, represent an effective strategy for the treatment of MM [2].
Clonal plasma cells express several antigens on their surface, with the most important molecule under investigation as potential targets for mAbs, such as CD38, CD40, CD138, CD56, CD54, PD1, kappa light chain, B-cell maturation antigen (BCMA) and the signaling lymphocyte activation molecule F7 (SLAMF7). To date, novel immunotherapies, including antibody–drug conjugates (ADC), bispecific antibodies and chimeric antigen receptor T-cell (CAR-T), represent an important option for relapsed or refractory disease resulting in high-quality, deep and durable responses [5].
More recently, based on the results of the pivotal phase III MAIA trial, daratumumab was approved, in combination with Rd, in newly diagnosed MM not eligible for ASCT [16][6]. Another daratumumab-based combination therapy has been recently approved, D-VMP, for newly diagnosed MM patients not eligible for ASCT [17][7].
Isatuximab, an IgG-k chimeric monoclonal antibody targeting a specific epitope on CD38, was investigated in combination with other drugs in relapsed/refractory settings. Based on the efficacy results, in terms of ORR and PFS, of the phase III ICARIA study, the combination of isatuximab plus Pd was approved for RRMM patients who have received at least two prior lines of therapy, including lenalidomide [19][8].
Selinexor, melfulflen, cerebron E3 ligase modulators (CELMoDs) and venetoclax represent very promising drugs for RRMM patients’ treatment.
The first-in-class oral selective inhibitor of nuclear export targeting exportin-1, selinexor, received FDA approval in combination with bortezomib and dexamethasone (SVd) for RRMM patients after the first line of therapy [23][9]. The median PFS for patients treated with SVd was 13.9 months compared to 9.5 months for patients treated with Vd. In addition, selinexor was approved in combination with dexamethasone for triple refractory patients [24][10]. Melfuflen (melfuflen-flufenamide) is the first-in-class peptide-drug conjugate targeting aminopeptidases and releasing alkylating agents into clonal plasma cells [25][11]. The phase II HORIZON trial demonstrated melfuflen efficacy in association with dexamethasone, with a median PFS of 4.2 months in a very heavily pretreated population [26][12].
Frequent, deep, and durable responses to BCMA-directed CAR-T cells (idecabtagene–vicleucel) have been recently reported in MM patients who were triple-class exposed and refractory to their last regimen in the multicenter pivotal phase II KarMMa-2 trial [29][13]. This has led to the rapid approval by FDA and by EMA of the first product (idecabtagene–vicleucel) for RR MM patients after three lines of therapy. On February 2022, FDA approved a second product, ciltacabtagene–autoleucel, for the treatment of RR MM patients after four or more lines of therapy, based on the results of the phase 1b/2 study CARTITUDE-1 [30][14].
2. Belantamab Mafodotin
Belantamab Mafodotin is the first-in-class ADC targeting BCMA that obtained FDA accelerated approval in August 2020, based on the results of phase II DREAMM-2 trial, for patients with RRMM who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody [32][15]. On November 2022, FDA announced that the process for the withdrawal of the US marketing authorization for belantamab mafodotin had been initiated. This action is based on the results of the DREAMM-3 trial (NCT04162210), in which the primary endpoint of PFS was not met, with a hazard ratio of 1.03 (95% confidence interval, 0.72–1.47) in a head-to-head comparison of belantamab mafodotin versus Pd.2.1. Mechanism of Action
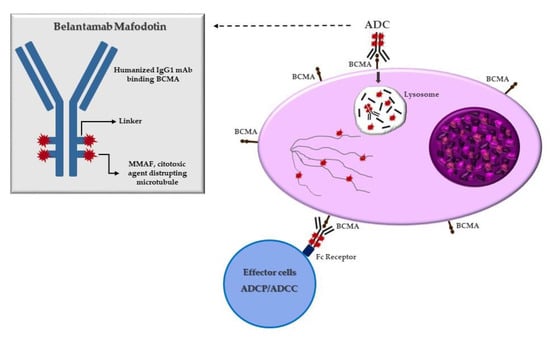
ADCs, an evolution of naked mAbs, consist of a mAb attached to a specific cytotoxic payload covalently conjugated through chemical linkers. The mechanism of action of ADCs is unique, whereby the mAb binds a tumor-specific antigen on clonal plasma cells resulting in cytotoxic payload internalization. Once the ADCs are intracellular, lysosomal degradation occurs, causing the release of the toxic payload within the plasma cells. Then, the free toxic payload enters the cytoplasm and/or nucleus, exerting its effect and causing apoptosis and cell death [33][16]. BCMA is a member of the TNF receptor superfamily and can be an optimal target for ADCs in MM due to its high expression on clonal plasma cells. Indeed, it is a cell-surface receptor protein expressed almost in end-stage B lymphocytes and clonal plasma cells. Because of its unique expression on MM cells, BCMA has become of interest to developing specifically targeted immunotherapies for MM. During the malignant transformation of immature plasma cells, the BCMA receptor and its ligand, named a proliferation-inducing ligand (APRIL) and B-cell activating factor (BAFF), overexpression activate several signal transduction pathways involved in oncogenesis, including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), protein kinase B (AKT), signal transducer and activator of transcription 3 (STAT3), phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinases (MAPK) cascades. The most common naked mAbs used in MM treatment are humanized antibodies or fully human immunoglobulin G subtype thanks to its long-circulating half-life in the bloodstream and lower immunogenicity. For ADCs, the choice of the ideal antibody is important. However, for the choice of ideal ADCs, it is important, beyond the antibody structure, the covalent linker because, as mentioned, linkers play a crucial role in releasing the potent drug at target tumor cells. Indeed, it has to be able to avoid premature degradation in the plasma because it can cause the release of cytotoxic payload with off-target effects on healthy cells. But, at the same time, it has to be able to degrade the cytotoxic component in the pathologic cells once the ADC can be internalized in the malignant target cell [33][16]. ADCs linkers are classified according to different categories in terms of the mechanism of drug release and their stability in circulation, including cleavable linkers and non-cleavable linkers. Cleavable linkers are designed to respond to a specific physiological environment, such as there being high glutathione concentrations, low pH, and special protease, which could assist the linkers in enabling chemical or biochemical reactions by way of hydrolyzation or proteolysis. The second group is non-cleavable linkers that rely on the monoclonal antibody degradation after ADCs’ internalization within the lysosomes and endosomes to generate the metabolites containing the active cytotoxic drugs with or without a portion of the linkers [35][17]. Belantamab mafodotin, also called belamaf (GSK2587916), is the first-in-class humanized IgG1 ADC that targets BCMA, approved for RRMM patients after four prior lines of therapy. This ADC is composed of an antibody that is able to bind BCMA, conjugated to a cytotoxic agent, monometil auristatin F (mafodotin), by a protease-resistant maleimidocaproyl linker. After binding BCMA on target cells, Belamaf is internalized and undergoes a process of proteolytic cleavage, releasing mafodotin. The released mafodotin disrupts the microtubular cell network leading to cell cycle arrest and apoptosis [32][15] (Figure 1).
Figure 1. Mechanism of action of belantamab mafodotin. After binding to BCMA on plasmacell, ADC is degraded in the lysosome with release of MMAF that leads to G2/M arrest and caspase 3 dependent apoptosis. Belamaf exerts antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
Belantamab mafodotin, also called belamaf (GSK2587916), is the first-in-class humanized IgG1 ADC that targets BCMA, approved for RRMM patients after four prior lines of therapy. This ADC is composed of an antibody that is able to bind BCMA, conjugated to a cytotoxic agent, monometil auristatin F (mafodotin), by a protease-resistant maleimidocaproyl linker. After binding BCMA on target cells, Belamaf is internalized and undergoes a process of proteolytic cleavage, releasing mafodotin. The released mafodotin disrupts the microtubular cell network leading to cell cycle arrest and apoptosis [32][15] (Figure 1).


Figure 1. Mechanism of action of belantamab mafodotin. After binding to BCMA on plasmacell, ADC is degraded in the lysosome with release of MMAF that leads to G2/M arrest and caspase 3 dependent apoptosis. Belamaf exerts antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
2.2. Dosing and Administration
The recommended dose for belantamab mafodotin is 2.5 mg/kg, administered once every 3 weeks and continued until disease progression or unacceptable toxicity. No dose modification is required for renal impairment if the estimated glomerular filtration rate (eGFR) is >30 mL/min/1.73 m2 or for mild hepatic impairment where total bilirubin is ≤1.5 times the upper limit of normal. However, no dosing recommendations have been established for patients with eGFR < 30 mL/min/1.73 m2, those with end-stage renal disease either with or without dialysis, or those with moderate to severe hepatic impairment. Therefore, due to the lack of safety and efficacy data for severe renal and hepatic impairment at this time, belamaf should be considered contraindicated in these patients. Dose interruption, reduction or discontinuation may be required for adverse events such as thrombocytopenia, infusion reactions or ocular adverse events.2.3. Toxicities
The most important adverse event related to belamaf therapy is ocular toxicity as reported by DREAMM-1 and DREAMM-2 clinical trials. Ocular events include keratopathy (defined as corneal epithelial changes named microcyst-like epithelial changes, MECs), best-corrected visual activity (BCVA) reduction and any other ocular symptoms, such as blurred vision, dry eye and corneal ulceration [32][15]. ADC adverse events could be explained by on-target or off-target mechanisms: indeed, considering that the majority of the proteins targeted by these agents are not expressed in the cornea (such as BCMA), ocular events may represent a specific off-target mechanism. Microtubule-disrupting monomethylauristatin-F (MMAF) is the cytotoxic component of Belamaf that is linked to a monoclonal antibody via protease-resistant maleimidocaproyl (mc) linker. MMAF is proposed as an attributable cause of ocular toxicity along with other ADCs that use the MMAF. Several factors are involved in promoting off-target ocular toxicity by ADCs, such as linker instability or premature cleavage in extracellular environments, linker-cytotoxin intracellular metabolism, and Fc-receptor-mediated cellular uptakes. Belamaf induces apoptosis of myeloma cells but may cause concomitant off-target apoptosis of corneal epithelial cells due to microtubulin inhibition caused by MMAF. In part-1 of the DREAMM-1 trial, where belamaf dose ranged from 0.03 mg/kg to 4.60 mg/kg, the ocular toxicity occurred more frequently at a larger dose than a smaller dose [41][18]. In the pivotal phase II DREAMM-2 study, keratopathy was the most common ocular toxicity (73%) irrespective of its grades (71% in 2.5 mg/kg versus 75% in 3.4 mg/kg) and the most common complaints were also blurred vision (22% for 2.5 mg/kg versus 30% for 3.4 mg/kg) and dry eyes (14% for 2.5 mg/kg versus 23% for 3.4 mg/kg). When corneal changes occur in the form of keratopathy, most patients are symptomatic. Lin et al. [42][19] analyzed imipramine as a new potential drug against macropinocytosis in cellular and biological systems, so theoretically, the inhibition of belamaf macropinocytosis might reduce the occurrence of ocular toxicity, but the practical role of such inhibition is limited [44][20]. To date, it has been demonstrated that dose delay or dose reduction are the only actions to reduce ocular toxicity, in order to allow appropriate time for replacement of corneal epithelial cells. However, no specific guidelines are yet available to apply effective dose delay or reduction. Several trials are ongoing to evaluate alternative dose-reduction strategies. DREAMM-2 reported that treatment delay (more than 63 days) did not negatively impact belantamab mafodotin efficacy. Of 16 patients, 38% deepened their response; 38% maintained the same response, and 13% showed an increase of serum monoclonal protein in the absence of criteria for disease progression [45][21]. In the DREAMM-2 study, the median time on therapy was 2.1 months (0.5–41 months) and 2.8 months (0.5–42.8) for patients randomized to 2.5 mg/kg and 3.4 mg/kg, respectively. As per the safety profile, grade ≥ 3 adverse events occurred in 84% of patients receiving belamaf 2.5 mg/kg and 83% of those treated with 3.4 mg/kg, requiring dose reduction in 36% and 44% and permanent discontinuation of belamaf in 9% and 5% of patients, respectively [46][22]. The main grade ≥ 3 hematologic toxicities were anemia occurring in 21% and 28% of 2.5 mg/kg and 3.4 mg/kg cohorts, whereas thrombocytopenia was documented in 19% and 29% of patients, respectively. Among nonhematologic adverse events, the most common was ocular toxicity, whose rate observed in patients allocated to 2.5 mg/kg was similar to that of the 3.4 mg/kg group. In the first cohort, any grade keratopathy occurred in 71% of patients (grade ≥ 3 = 29%), blurred vision in 25% and BCVA reduced to 20/50 or worse in 48% of patients. The median time to keratopathy resolution was 120 days, whereas the median time to resolution of the first BCVA event was 23 days. However, only 3% of patients in both study arms permanently discontinued treatment because of ocular events [46][22].3. Clinical Trials on Belantamab Mafodotin
3.2. Ongoing Studies with Belantamab Mafodotin-Based Regimens in Relapsed/Refractory MM
3.1. Ongoing Studies with Belantamab Mafodotin-Based Regimens in Relapsed/Refractory MM
Phase I/II Algonquin study evaluated the safety and efficacy of different doses and schedules of belamaf combined with Pd (Bela-Pd) in RRMM who had received at least one prior line of therapy, exposed to lenalidomide and PI and pomalidomide naïve [49][23]. In Part 1 of the study, a dose-escalation phase, patients were treated with pomalidomide 4 mg days 1–21, dexamethasone 40 mg weekly and belamaf as a single dose of 1.92 mg/kg every 4 weeks, as a single dose of 2.5 mg/kg every 4 weeks, every 8 weeks or every 12 weeks, or split on days 1 and 8 (2.5 mg/kg or 3.4 mg/kg) every 4 weeks. Considering the 54 triple-class exposed patients enrolled in the study, the median age was 67.5 years, the median number of prior lines of therapy was 3 (range 2–5) and 72.2% were triple-refractory. Across all cohorts, ORR was 86%, and 60% of patients achieved at least a VGPR. As per outcome measures, after a median follow-up of 5.7 months, the median PFS resulted in being 15.6 months, an impressive result considering that in the prospective LocoMMotion study, triple-class exposed patients treated with standard of care had a median PFS of 4.6 months [22][24]. Most common grade ≥ 3 adverse events included keratopathy (55%), neutropenia (37%), thrombocytopenia (27.5%) and decreased BCVA (23.5%). Other studies with belamaf also including patients with early relapse are ongoing. Phase I/II DREAMM-6 study is exploring the safety and activity of up to 3 dose levels and up to dosing schedules of belamaf in combination with lenalidomide/dexamethasone (Rd, arm A) or bortezomib/dexamethasone (Vd, arm B) in patients with ≥1 prior line of therapy. Preliminary results of 18 patients with a median of 3 prior lines of therapy (range 1–11) enrolled in arm B and receiving belamaf 2.5 mg/kg single dosing plus Vd showed an ORR of 78% with 67% of patients achieving at least VGPR. After a median of 25.5 weeks on treatment, the median DoR was not reached. Grade ≥ 3 thrombocytopenia occurred in 66% of patients, grade 3 keratopathy in 61%, whereas peripheral neuropathy (all grade ≤ 2) was observed in 33% of patients [45][21].3.3. Ongoing Studies with Belantamab Mafodotin-Based Regimens in Other Cancers
3.2. Ongoing Studies with Belantamab Mafodotin-Based Regimens in Other Cancers
EMN27 phase 2 trial is recruiting patients with relapsed refractory AL Amyloidosis to receive Belantamab mafodotin at the same dose approved in MM, with the primary endpoint of CR/VGPR (NCT04617925). Another phase 1/2a study, not yet recruiting, has been built to enroll relapsed patients with AL amyloidosis (NCT05145816). A phase 1 trial is evaluating Belantamab mafodotin for the treatment of high-risk Smoldering Myeloma (NCT05055063). Belantamab mafodotin is on the study also in relapsed plasmablastic lymphoma and ALK+ large B cell lymphoma in a phase 2 trial (NCT04676360). Data are awaited.3.4. Sequencing of Belantamab Mafodotin with Other BCMA-Targeting Immunotherapies
3.3. Sequencing of Belantamab Mafodotin with Other BCMA-Targeting Immunotherapies
The availability of anti-BCMA immunotherapies as CAR-T cells products ide-cel [29][13] and cilta-cel [50][25], as well as the approval by FDA and EMA of bispecific antibody teclistamab [31][26], raised the question of the effectiveness of retreatment with another product in patients already exposed to BCMA targeting immunotherapies. A recent analysis, using data from DREAMM-1 and DREAMM-2 trials, evaluated free sBCMA concentration at baseline, at the achievement of best response and, at the latest, progression in order to study BCMA expression during treatment with belamaf [51][27].3.5. Belantamab Mafodotin in Newly Diagnosed MM
3.4. Belantamab Mafodotin in Newly Diagnosed MM
As well as for other novel immunotherapies, several clinical trials are exploring belamaf in the upfront setting. At the last ASH Meeting, preliminary results from the phase II Spanish GEM-BELA-VRd trial were presented [54][28]. Treatment included six induction cycles with VRd plus belamaf 2.5 mg/kg every 8 weeks, high dose melphalan and ASCT, followed by consolidation with two cycles with Bela-VRd and maintenance with lenalidomide until progression plus belamaf for 2 years. Among the 40 patients who received at least four cycles of induction, ORR was 82%, at least VGPR 69% and CR 13%. Ocular toxicity represented the most common toxicity since 77.5% of patients developed blurred vision and 60% any grade keratopahy. Neutropenia and thrombocytopenia were the most frequent hematologic adverse events.4. Real Life Data on Belantamab Mafodotin
4.1. USA Real-Life Experiences
Several American real-life experiences have been published in order to better define the efficacy and toxicity of belantamab outside clinical trials, which had restricted patients’ selection, risking providing poorly reproducible data in real-life settings. Vaxman et al. [56][29] retrospectively identified 36 MMRR patients who received at least one dose of belantamab outside a clinical trial at all three Mayo Clinic sites, from September 2020 to June 2021, with a median follow-up of 6 months. Patients’ median prior lines of therapy were eight, more than DREAMM-2 trial, whose six patients (17%) received belantamab in combination with other agents (pomalidomide, cyclophosphamide and thalidomide) and seven patients (19%) had already received another anti BCMA agent (CART) prior to belantamab therapy. These features characterized a heavily pretreated population, also BCMA-refractory, differently from the pivotal study. The median time from diagnosis to the first belantamab dose was 7 years. They reported an ORR of 33% with a median PFS and OS for the whole cohort of 2 months and 6.5 months, respectively. Eighteen patients died, all for disease progression. More recently, Abeykoon et al. [57][30] conducted a retrospective observational study on 38 RRMM patients treated with belantamab at Mayo Clinic between January 2020 and January 2021, with a median follow-up of 11 months, to evaluate the impact of belantamab mafodotin-induced ocular toxicity on the outcomes of patients themselves. Authors found a 29% ORR, with a median PFS and OS of 2 and 7.2 months, respectively, in a really heavily pretreated population (median prior lines of therapy 8, range 2–15), with 89% of patients having high-risk disease characteristics and a median time from diagnosis to belamaf start of 7 years (range 1–19.7). Ocular toxicity was observed in 27 (75%) patients: keratopathy in 25 (69%), decreased BCVA in 21, and/or ocular symptoms like xerophthalmia in 13 (36%). Ocular toxicity seemed to develop earlier in responders patients than in non-responders. Becnel et al. [58][31] presented at ASCO 2022 data of a retrospective, single-center, real-world experience of belantamab mafodotin in 39 RRMM patients treated between November 2020 and November 2021 at MD Anderson Cancer Center in Houston. The overall population had seven median prior lines of therapy (range 3–16) and 38% of whom had high-risk FISH features, 38% extramedullary disease and 69% did not meet eligibility criteria for the DREAMM-2 study, picturing a more difficult population to treat than the pivotal study. Moreover, eight patients were even anti-BCMA-refractory; one of them obtained a PR and another an MR. The authors found a 27% ORR with a CBR of 35%. Median PFS was 1.8 months and median OS 9.2 months, with a not reached median DOR at a median follow-up of 10.1 months.4.2. Asian Real-Life Experience
Shragai et al. published data from an Israelian observational multicenter real-life study treating 106 RRMM patients with belantamab between 2019 and 2021, during the compassionate program, with or without steroids. Baseline population characteristics were similar to the DREAMM-2 study, but patients had lower median prior lines of therapy (6 vs. 7). Differently from the pivotal study, there was 32% of penta-refractory patients, and the median age was a little higher (69.4 vs. 65 years). ORR was 45.5%, and this rate was maintained among different subgroups (age, sex, triple-or penta-refractoriness, ISS, R-ISS, high-risk cytogenetics, EMD). At a median follow-up of 11.9 months, the median PFS was 4.7 months in the whole population, while it was 8.8 months in responders, confirming the significant association between response deepness and outcomes already reported in DREAMM-2. There was no difference in PFS between the whole cohort and triple-refractory or penta-refractory patients. Median DOR was 8.1 months and median OS 14.5 months, without differences based on cytogenetic risk or refractoriness status, but better in responders than not responders. As for safety, the most common adverse event was ocular; 65 (68.4%) patients experimented with keratopathy, of whom 63.4% resolved to grade 1 or less.4.3. European Real-Life Experiences
Offidani et al. reported data from an observational, multicenter, retrospective real-life study on 67 RRMM patients treated with belantamab in compassionate use programs such as Named Patient Program (NPP) and Expanded Access Program (EAP) in different Italian centers under the aegis of European Myeloma Network (EMN). Compared to DREAMM-2, this cohort had fewer previous lines of therapy (5 vs. 7) but similar median age with similar general characteristics. Authors found similar ORR (31%) and CBR (37%). Median PFS was 3.7 months, median OS 12.9 months and median DOR 13.8 months; they seemed higher than the pivotal study. Authors confirmed that ocular toxicity was the most common, mostly grade < 3 (87%), with keratopathy reported in 23 (74%) patients, ocular symptoms in 5 (16%) and changes in BCVA in 3 (10%), all reversible during the follow-up.4.4. A Comparison of Different Real-Life Experiences
Despite there being no significant differences in efficacy or safety among different geographical settings, a trend of better outcomes could be identified in European and Asian experiences. These studies have sometimes reached a 35–40% of ORR even without an increase of PFS and DOR, probably because they are more recent and they enrolled fewer pretreated patients. As for safety, the discontinuation rate for toxicity, which could be considered the best representative parameter, remains often similar among different papers, describing a good safety profile of the drug even in real-life populations.5. Conclusions
The increasing number of patients receiving all classes of drugs as IMiDs, PIs and mAbs, acquires multi-refractoriness status and requires the development of novel therapeutic strategies that try to overcome the clonal complexity and heterogeneity of MM. Additionally, naked mAbs, among new immunotherapies, Belantamab Mafodotin has been the first-in-class anti-BCMA ADC to be approved for advanced RRMM. In the DREAMM-2 study [32][15], belamaf monotherapy was able to induce substantial ORR in patients who had received more than five prior lines of therapy, showing activity either in standard or in high-risk RRMM patients [46][22], with a remission duration of quite one year. Notably, these results have also been confirmed in real-world experiences. Impressive anti-myeloma activity has been documented with bispecific antibodies and CAR T cell therapies, but these immunotherapies are not yet available in many countries and, concerning CAR T cells, they required personalized manufacturing time with a median period from leukapheresis to infusion for ide-cel of 40 days [29][13], making this therapy challenging in patients with rapidly progressive disease. Other limitations should be taken into account as the presence of an adequate composition of T cell populations in the patients, the need for bridging therapy and good performance status, the need for family support and, last but not least, the very high cost of therapy. Moreover, both CART cells and bispecific antibodies such as teclistamab, the only one approved for RRMM, necessitate hospitalization to manage possible early severe toxicities such as CRS (cytokine release syndrome) and ICANS (immune effector cell associated neurotoxicity syndrome). The main advantages of using belamaf in triple-refractory patients are the immediate availability and no need for hospitalization. Moreover, while retreatment with anti-CD38 mAb is not effective, the other anti-BCMA immunotherapies can be administered after belamaf leading to a significant duration of response in very heavily pretreated MM patients [52][32]. Ocular toxicity represents a peculiar side effect of belamaf, but close monitoring and collaboration between hematologists and ophthalmologists are making this toxicity easier to predict and manage. Several ongoing studies are evaluating different belamaf doses and schedules in order to reduce its incidence. In RRMM, the results of trials exploring belamaf in combination with IMiDs and PIs, as well as those including very innovative agents, are expected since these potential new regimens could represent an exciting step for belamaf to address unmet needs in MM paradigm.References
- Abramson, H.N. Monoclonal Antibodies for the Treatment of Multiple Myeloma: An Update. Int. J. Mol. Sci. 2018, 19, 3924.
- Bonello, F.; Mina, R.; Boccadoro, M.; Gay, F. Therapeutic Monoclonal Antibodies and Antibody Products: Current Practices and Development in Multiple Myeloma. Cancers 2020, 12, 15.
- Wudhikarn, K.; Wills, B.; Lesokhin, A.M. Monoclonal Antibodies in Multiple Myeloma: Current and Emerging Targets and Mechanisms of Action. Best Pract. Res. Clin. Haematol. 2020, 33, 101143.
- Kumar, S.K.; Therneau, T.M.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Rajkumar, S.V.; Fonseca, R.; Witzig, T.E.; Lust, J.A.; Larson, D.R.; et al. Clinical Course of Patients with Relapsed Multiple Myeloma. Mayo Clin. Proc. 2004, 79, 867–874.
- Mikkilineni, L.; Kochenderfer, J.N. Chimeric Antigen Receptor T-Cell Therapies for Multiple Myeloma. Blood 2017, 130, 2594–2602.
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115.
- Mateos, M.-V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528.
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus Pomalidomide and Low-Dose Dexamethasone versus Pomalidomide and Low-Dose Dexamethasone in Patients with Relapsed and Refractory Multiple Myeloma (ICARIA-MM): A Randomised, Multicentre, Open-Label, Phase 3 Study. Lancet 2019, 394, 2096–2107.
- Grosicki, S.; Simonova, M.; Spicka, I.; Pour, L.; Kriachok, I.; Gavriatopoulou, M.; Pylypenko, H.; Auner, H.W.; Leleu, X.; Doronin, V.; et al. Once-per-Week Selinexor, Bortezomib, and Dexamethasone versus Twice-per-Week Bortezomib and Dexamethasone in Patients with Multiple Myeloma (BOSTON): A Randomised, Open-Label, Phase 3 Trial. Lancet 2020, 396, 1563–1573.
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor–Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738.
- Chauhan, D.; Ray, A.; Viktorsson, K.; Spira, J.; Paba-Prada, C.; Munshi, N.; Richardson, P.; Lewensohn, R.; Anderson, K.C. In Vitro and in Vivo Antitumor Activity of a Novel Alkylating Agent, Melphalan-Flufenamide, against Multiple Myeloma Cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 3019–3031.
- Richardson, P.G.; Oriol, A.; Larocca, A.; Bladé, J.; Cavo, M.; Rodriguez-Otero, P.; Leleu, X.; Nadeem, O.; Hiemenz, J.W.; Hassoun, H.; et al. Melflufen and Dexamethasone in Heavily Pretreated Relapsed and Refractory Multiple Myeloma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 757–767.
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716.
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma (CARTITUDE-1): A Phase 1b/2 Open-Label Study. Lancet 2021, 398, 314–324.
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab Mafodotin for Relapsed or Refractory Multiple Myeloma (DREAMM-2): A Two-Arm, Randomised, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 207–221.
- Tsuchikama, K.; An, Z. Antibody-Drug Conjugates: Recent Advances in Conjugation and Linker Chemistries. Protein Cell 2018, 9, 33–46.
- Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Linkers Having a Crucial Role in Antibody-Drug Conjugates. Int. J. Mol. Sci. 2016, 17, 561.
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody–Drug Conjugate, GSK2857916, in Relapsed/Refractory Multiple Myeloma: An Update on Safety and Efficacy from Dose Expansion Phase I Study. Blood Cancer J. 2019, 9, 37.
- Ferron-Brady, G.; Rathi, C.; Collins, J.; Struemper, H.; Opalinska, J.; Jewell, R.C. Exposure-Response (E-R) for Ocular Safety Endpoints for Belantamab Mafodotin (Belamaf), a B-Cell Maturation Antigen (BCMA)-Targeting Agent, in Patients with Relapsed/Refractory Multiple Myeloma (RRMM) in the DREAMM-2 Study. Blood 2020, 136, 20–21.
- Lin, H.-P.; Singla, B.; Ghoshal, P.; Faulkner, J.L.; Cherian-Shaw, M.; O’Connor, P.M.; She, J.-X.; de Chantemele, E.J.; Csányi, G. Identification of Novel Macropinocytosis Inhibitors Using a Rational Screen of Food and Drug Administration-Approved Drugs. Br. J. Pharmacol. 2018, 175, 3640–3655.
- Popat, R.; Nooka, A.; Stockerl-Goldstein, K.; Abonour, R.; Ramaekers, R.; Khot, A.; Forbes, A.; Lee, C.; Augustson, B.; Spencer, A.; et al. DREAMM-6: Safety, Tolerability and Clinical Activity of Belantamab Mafodotin (Belamaf) in Combination with Bortezomib/Dexamethasone (BorDex) in Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 19–20.
- Nooka, A.K.; Cohen, A.; Lee, H.C.; Badros, A.Z.; Suvannasankha, A.; Callander, N.; Abdallah, A.-O.; Trudel, S.; Chari, A.; Libby, E.; et al. Single-Agent Belantamab Mafodotin in Patients with Relapsed or Refractory Multiple Myeloma: Final Analysis of the DREAMM-2 Trial. Blood 2022, 140, 7301–7303.
- Trudel, S.; McCurdy, A.; Fu, M.; Sutherland, H.J.; Louzada, M.L.; Chu, M.P.; White, D.J.; Mian, H.S.; Kotb, R.; Othman, I.; et al. Belantamab Mafodotin in Combination with Pomalidomide and Dexamethasone Demonstrates Durable Responses in Triple Class Exposed/Refractory Multiple Myeloma. Blood 2022, 140, 7306–7307.
- Mateos, M.V.; Weisel, K.; De Stefano, V.; Goldschmidt, H.; Delforge, M.; Mohty, M.; Cavo, M.; Vij, R.; Lindsey-Hill, J.; Dytfeld, D.; et al. LocoMMotion: A Prospective, Non-Interventional, Multinational Study of Real-Life Current Standards of Care in Patients with Relapsed and/or Refractory Multiple Myeloma. Leukemia 2022, 36, 1371–1376.
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Agha, M.; Cohen, A.D.; Hari, P.; Avigan, D.; Deol, A.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, an Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J. Clin. Oncol. 2022, 41, 1265–1274.
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.C.J.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505.
- Lowther, D.E.; Houseman, E.A.; Han, G.; Kleanthous, E.; Knoblock, D.; Zhou, X.; Banerjee, S.; Patel, S.; Figueroa, D. No Evidence of BCMA Expression Loss or Systemic Immune Impairment after Treatment with the BCMA-Targeted Antibody-Drug Conjugate (ADC) Belantamab Mafodotin (Belamaf) in the DREAMM-1 and DREAMM-2 Trials of Patients with Relapsed/Refractory Multiple Myeloma. Blood 2022, 140, 611–613.
- Gonzalez-Calle, V.; Rodriguez Otero, P.; Rey-Bua, B.; De La Rubia, J.; De Arriba, F.; Cabañas, V.; Garcia, E.G.; Ocio, E.M.; Encinas, C.; Suarez Cabrera, A.; et al. Belantamab Mafodotin in Combination with Vrd for the Treatment of Newly Diagnosed Transplant Eligible Multiple Myeloma Patients: Results from the Phase II, Open Label, Multicenter, GEM-BELA-Vrd Trial. Blood 2022, 140, 7286–7288.
- Vaxman, I.; Abeykoon, J.; Dispenzieri, A.; Kumar, S.K.; Buadi, F.; Lacy, M.Q.; Dingli, D.; Hwa, Y.; Fonder, A.; Hobbs, M.; et al. “Real-Life” Data of the Efficacy and Safety of Belantamab Mafodotin in Relapsed Multiple Myeloma—The Mayo Clinic Experience. Blood Cancer J. 2021, 11, 196.
- Abeykoon, J.P.; Vaxman, J.; Patel, S.V.; Kumar, S.; Malave, G.C.; Young, K.S.; Ailawadhi, S.; Larsen, J.T.; Dispenzieri, A.; Muchtar, E.; et al. Impact of Belantamab Mafodotin-Induced Ocular Toxicity on Outcomes of Patients with Advanced Multiple Myeloma. Br. J. Haematol. 2022, 199, 95–99.
- Becnel, M.; Ferreri, C.J.; Feng, L.; Richards, T.A.; Horowitz, S.B.; Patel, N.; Gombos, D.S.; Razmandi, A.; Murga, A.; Seif, S.; et al. Retrospective, Single-Center, Real-World Experience of Belantamab Mafodotin in Relapsed/Refractory Multiple Myeloma. J. Clin. Oncol. 2022, 40, 8060.
- Cohen, A.D.; Mateos, M.V.; Cohen, Y.C.; Rodriguez-Otero, P.; Paiva, B.; van de Donk, N.W.C.J.; Martin, T.; Suvannasankha, A.; De Braganca, K.C.; Corsale, C.; et al. Efficacy and Safety of Cilta-Cel in Patients with Progressive Multiple Myeloma after Exposure to Other BCMA-Targeting Agents. Blood 2023, 141, 219–230.
More
