Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Zoya Gridneva.
Intakes of human milk components have been associated with infant body composition, which is likely partially implicated in the reduced risk of developing childhood obesity among breastfed infants.
- systematic review
- human milk intake
- early life nutrition
- breastfeeding
1. Protein
Total protein was the most frequently studied HM macronutrient (7/10 studies, Figure 3, Table A1 and Table A2). Four out of seven studies (57%) found no significant relationships between total protein intake and infant anthropometry or BC [29,35,42,44][1][2][3][4]. Three studies found positive relationships with infant anthropometry [34[5][6],45], FFM [45][6], and adiposity [34,38][5][7]; however, one of these studies also reported a change in the direction of the relationship with infant adiposity (skinfold gain) from positive to negative from 3 months of age onwards [34][5].
Three studies reported on the effect of HM casein intake and concentration on infant anthropometry and BC [35,38,42][2][3][7]. Casein intake was positively associated with infant whole body adiposity (FM, %FM, and FMI) [42][3] as well as subcutaneous-abdominal adiposity [38][7] and negatively with FFM [42][3], but not with mid-arm and mid-thigh lean and fat areas [35][2]. The same three studies also analysed the effect of whey protein on infant BC outcomes and found no relationships after accounting for multiple comparisons.
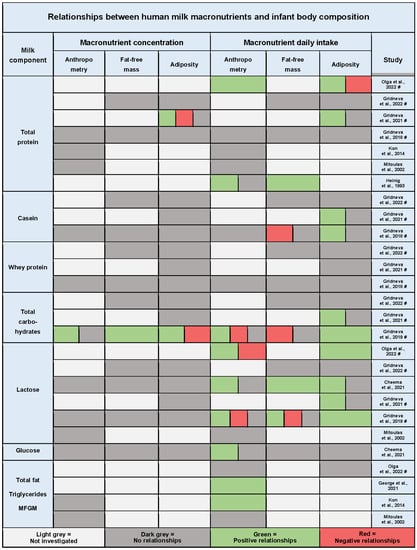
Figure 31. Summary of the results of quantitative synthesis for studies investigating relationships between human milk macronutrient intake and infant anthropometry, fat-free mass, and adiposity. Each cell represents one or multiple significant or non-significant results reported by the study. Significance level was determined by the study and, where multiple comparisons adjustment was performed (indicated by #), only results significant after the adjustment are presented; if no adjustment was performed, the results for p < 0.05 are presented. MFGM, milk fat globule membrane lipid species [29,34,35,37,38,39,41,42,44,45][1][2][3][4][5][6][7][8][9][10].
2. Carbohydrates
Six out of ten studies analysed relationships between HM lactose intake and BC outcomes [29,34,35,37,38,41] (Figure 3, Table A1)[1][2][5][7][8][10]. Most studies (67%, 4/6) found predominantly positive relationships between lactose intake and infant anthropometry [34[5][8][10],37,41], FFM [37[8][10],41], and adiposity parameters [34,37,38,41][5][7][8][10]. However, two studies reported a change in the direction of the relationship, similar to protein, from positive to negative. Negative relationships were found with infant BMI and FFMI between 5 and 12, at 12 months of age [41][10], and with weight gain after 3 months of age onwards [34][5]. No relationships with the concentration of lactose were shown.
In addition to lactose, three studies from the same cohort analysed associations between 24 h intake of HM total carbohydrates and infant anthropometry and BC with contradicting results. One study of the two that investigated regional BC did not find any associations with infant lean and fat limb areas [35][2], while a study of infant subcutaneous-abdominal adiposity reported positive associations with total carbohydrate intake [38][7]. The third study investigated whole BC and reported opposite associations for intakes and concentrations of total carbohydrates [41][10]. Infant FM associations were positive with total carbohydrate intake and negative with concentration, yet for FFM, the associations were negative with intake and positive with concentration.
Only one study investigated relationships between HM glucose intake and infant BC outcomes, reporting that a higher glucose intake was associated with an increased head circumference [37][8].
3. Fat
Four studies analysed 24 h HM fat intake relationships with infant anthropometry and BC (Figure 3, Table A1). Two longitudinal studies that measured total fat intake did not report any relationships with either anthropometry [29,34][1][5] or skinfold gains [34][5]. The third longitudinal study that assessed intakes of individual MFGM lipid species found a large number of time-dependent positive correlations for 99 of the 166 measured species with infant weight, head circumference, and WLZ, with head circumference relationships being the strongest [39][9]. Cross-sectionally, at 3 months postpartum, a higher 24 h total fat intake was found among high weight gain infants compared with low weight gain infants [44][4].
Studies assessing fat concentration in addition to intake did not establish any relationships with BC or growth [29,44][1][4]. It is of note that only one study calculated fat intake by sampling HM pre-/post-feed over 24 h [29][1], with the other three studies estimating fat intake based on two (morning and evening) pre-feed samples [39][9] or a single mid-stream [44][4] or post-feed sample [34][5]. As fat concentration progressively increases during a breastfeed [50][11], the results are unlikely to be representative of true intake, as demonstrated by George et al. [51][12].
4. Twenty-Four-Hour Intakes of Bioactive Molecules and Infant Body Composition
4.1. Metabolic Hormones
Positive relationships between intakes of metabolic hormones and infant BC and anthropometry were found in several studies (Figure 4, Table A3).
Four studies investigated 24 h intake of HM adiponectin [35,38,43,44][2][4][7][13]. Cross-sectionally, at 3 months postpartum, skim milk adiponectin intake was higher in infants with high weight gain compared with the low weight gain group [44][4]. In a longitudinal cohort, whole milk adiponectin intake was significantly associated with infant whole BC, positively with adiposity, and negatively with FFM [43][13]. After adjusting for multiple comparisons, no significant associations with infant regional BC (visceral and subcutaneous-abdominal adiposity [38][7], as well as mid-arm and mid-thigh lean and fat areas [35][2]) were found.
The four studies that investigated adiponectin also measured HM leptin [35,38,43,44][2][4][7][13]. Two of the studies, which measured leptin in whole milk, found daily intakes were positively associated with infant whole body adiposity (higher leptin intakes at 12 months were associated with increased changes in infant FM and %FM between 2 and 12 months) [43][13]. No association was shown with visceral and subcutaneous-abdominal adiposity [38][7]. One study measured leptin in both whole and skim milk, with multiple skim milk leptin associations contrasting the whole milk leptin results [43][13]. In the cross-sectional study, higher skim milk leptin intakes were found in infants with high weight gain compared with the normal weight gain group at 2 months, while skim milk leptin concentrations were significantly higher in milk consumed by the infants with normal weight gain compared with the low weight gain group at 3 months of lactation [44][4].
The cross-sectional study was also the only one to assess HM IGF-1 and found, at 3 months postpartum, milk with a higher IGF-1 concentration was consumed by infants with high weight gain compared with the low weight gain group, and higher intakes IGF-1 were found in the groups of infants with high and normal weight gain compared with the low weight gain group [44][4].
The same study [44][4] also reported that, at 1 month postpartum, HM ghrelin concentrations were significantly higher in milk consumed by infants with high weight gain compared with those with normal weight gain, and by infants with normal weight gain compared with those with low weight gain. However, there was no difference between groups by infant intake of ghrelin at any lactation time points.
HM insulin intake was also assessed by one study, reporting no relationships with infant BC or anthropometry [37][8].
4.2. Immunomodulatory Proteins
Three studies from the same cohort analysed relationships between intakes of HM lactoferrin, lysozyme, and secretory immunoglobulin A (sIgA) and infant BC development during the first 12 months of lactation [35,38,40] (Figure 4, Table A3)[2][7][14].
Higher HM lysozyme intake during the first 12 months of lactation was associated with increased whole body adiposity (FM, FMI), while higher lysozyme intake at 12 months of age was associated with a decrease in infant FFMI between 5 and 12 months [40][14]. The association of lysozyme intake with infant mid-arm fat areas was time-dependent, positive at 2, 5, and 9 months of age and negative at 12 months [35][2]. After adjusting for multiple comparisons, no association was reported between lysozyme intake and visceral and subcutaneous-abdominal fat areas [38][7] or between lysozyme concentration and whole BC [40][14].
Increased HM lactoferrin intake during the first 12 months was associated with a decrease in infant FFMI [40][14], with no significant relationships with infant regional adiposity [35,38][2][7] or regional lean mass [35][2]. Lactoferrin concentration was positively associated with infant visceral depth at 5 and 9 months and negatively at 2 and 12 months [38][7].
After adjusting for multiple comparisons, no significant associations with either intake or concertation of HM sIgA were reported [35,38,40][2][7][14].
4.3. Human Milk Oligosaccharides
Four studies investigated either combined total (calculated as a subtraction of lactose concentration from total carbohydrate concentration) [35,38,41][2][7][10] or multiple individual HMOs [36][15] and found relationships with infant BC (Figure 4, Table A3).
Three studies from the same longitudinal cohort analysed relationships between intakes of total HMOs and infant whole [41][10] and regional [35,38][2][7] BC. After adjusting for multiple comparisons, no associations between intake of total HMOs and infant FFM or FM parameters were found, yet a higher total HMO concentration was associated with greater infant FFM and FFMI, and decreased FMI, %FM, and FM/FFM ratio at 5, 9, and 12 months (increased at 2 months) [41][10]. Total HMO intake did not relate to infant visceral and subcutaneous-abdominal adiposity [38][7], but was time-dependently associated with infant mid-arm fat area [35][2], negatively at 2 and positively at 5, 9, and 12 months of age.
In a recent larger cross-sectional study, positive associations were shown between intakes of six individual HMOs (2′-fucosyllactose (2′FL), 3-fucosyllactose (3FL), difucosyllactose (DFLac), difucosyllacto-N-hexaose (DFLNH), difucosyllacto-N-tetrose (DFLNT), and sialyl-lacto-N-tetraose (LSTb)) and multiple infant BC (FFM, FM) and anthropometry measures [36][15]. However, while the direction of intake relationships was positive, the concentration relationships did not match the intakes for all but one HMO (DFLNH), being either absent (2′FL and DFLac) or negative (ucosyllacto-N-hexaose (FLNH), lacto-N-neotetraose (LNnT), and lacto-N-fucopentaose III (LFNP III)). Additionally, relationships with infant BC were dependent on maternal secretor status [36][15].
References
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.A.; Sherriff, J.L.; Hartmann, P.E. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br. J. Nutr. 2002, 88, 29–37.
- Gridneva, Z.; Rea, A.; Lai, C.T.; Tie, W.J.; Kugananthan, S.; Warden, A.H.; Perrella, S.L.; Murray, K.; Geddes, D.T. Human milk macronutrients and bioactive molecules and development of regional fat depots in Western Australian infants during the first 12 months of lactation. Life 2022, 12, 493.
- Gridneva, Z.; Tie, W.J.; Rea, A.; Lai, C.T.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human milk casein and whey protein and infant body composition over the first 12 months of lactation. Nutrients 2018, 10, 1332.
- Kon, I.Y.; Shilina, N.M.; Gmoshinskaya, M.V.; Ivanushkina, T.A. The study of breast milk IGF-1, leptin, ghrelin and adiponectin levels as possible reasons of high weight gain in breast-fed infants. Ann. Nutr. Metab. 2014, 65, 317–323.
- Olga, L.; Vervoort, J.; van Diepen, J.A.; Gross, G.; Petry, C.J.; Prentice, P.M.; Chichlowski, M.; von Tol, E.A.F.; Hughes, I.A.; Dunger, D.B.; et al. Associations between breast milk intake volume, macronutrient intake, and infant growth in a longitudinal birth cohort: The Cambridge Baby Growth and Breastfeeding Study (CBGS-BF). Br. J. Nutr. 2022, 1–27.
- Heinig, M.J.; Nommsen, L.A.; Peerson, J.M.; Lonnerdal, B.; Dewey, K.G. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: The DARLING Study. Am. J. Clin. Nutr. 1993, 58, 152–161.
- Gridneva, Z.; Rea, A.; Lai, C.T.; Tie, W.J.; Kugananthan, S.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Development of visceral and subcutaneous-abdominal adipose tissue in breastfed infants during first year of lactation. Nutrients 2021, 13, 3294.
- Cheema, A.S.; Stinson, L.F.; Rea, A.; Lai, C.T.; Payne, M.S.; Murray, K.; Geddes, D.T.; Gridneva, Z. Human milk lactose, insulin, and glucose relative to infant body composition during exclusive breastfeeding. Nutrients 2021, 13, 3724.
- George, A.D.; Gay, M.C.L.; Selvalatchmanan, J.; Torta, F.; Bendt, A.K.; Wenk, M.R.; Murray, K.; Wlodek, M.E.; Geddes, D.T. Healthy breastfeeding infants consume different quantities of milk fat globule membrane lipids. Nutrients 2021, 13, 2951.
- Gridneva, Z.; Rea, A.; Tie, W.J.; Lai, C.T.; Kugananthan, S.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Carbohydrates in human milk and body composition of term infants during the first 12 months of lactation. Nutrients 2019, 11, 1472.
- Khan, S.; Hepworth, A.R.; Prime, D.K.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Variation in fat, lactose, and protein composition in breast milk over 24 hours: Associations with infant feeding patterns. J. Hum. Lact. 2013, 29, 81–89.
- George, A.D.; Gay, M.C.L.; Murray, K.; Muhlhausler, B.S.; Wlodek, M.E.; Geddes, D.T. Human milk sampling protocols affect estimation of infant lipid intake. J. Nutr. 2020, 150, 2924–2930.
- Gridneva, Z.; Kugananthan, S.; Rea, A.; Lai, C.T.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human milk adiponectin and leptin and infant body composition over the first 12 months of lactation. Nutrients 2018, 10, 1125.
- Gridneva, Z.; Lai, C.T.; Rea, A.; Tie, W.J.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human milk immunomodulatory proteins are related to development of infant body composition during the first year of lactation. Pediatr. Res. 2020, 89, 911–921.
- Cheema, A.S.; Gridneva, Z.; Furst, A.J.; Roman, A.S.; Trevenen, M.L.; Turlach, B.A.; Lai, C.T.; Stinson, L.F.; Bode, L.; Payne, M.S.; et al. Human milk oligosaccharides and bacterial profile modulate infant body composition during exclusive breastfeeding. Int. J. Mol. Sci. 2022, 23, 2865.
More
