2. Biological Mechanisms of Brain Metastasis
Systemic metastases are the result of a multi-stage process that involves the detachment of the neoplastic cells from the primary tumor mass, and their migration through the local mesenchyme into the blood and lymphatic vessels
[12]. This process requires reciprocal interactions between tumor cells and host tissues that involve epithelial-to-mesenchymal transition (EMT), changes in adhesion, proteolysis, invasion, and angiogenesis
[13][14][13,14].
In BC, these events are linked to the alteration of the Wnt signaling pathway and inactivation of cadherin 1 (CDH1)
[15][16][17][15,16,17]. Once neoplastic cells reach the peritumoral microenvironment, their survival relies on the so-called intravasation, which is the ability to invade the systemic circulation and reach distant anatomical sites
[18]. The brain is a richly vascularized organ and is therefore exposed to a high amount of circulating tumor cells, provided that they can cross the BBB
[19]. This barrier is composed of a layer of specialized endothelial cells expressing a specific subset of membrane transporters and pumps that allow tight regulation of the brain microenvironment and are impermeable to most foreign agents
[20]. Astrocytes further contribute to the regulation of the BBB by forming a second basement membrane around brain capillaries through their cytoplasmic foot processes
[21]. Still, some BC cells can cross the BBB and invade the brain parenchyma.
Colonization of the brain by BC starts with the adhesion of the circulating tumor cells to the capillary endothelium
[22][23][22,23]. Upregulation of the membrane glycosyl-transferase ST6GALNAC5 has been demonstrated to play an important role in this process
[24]. Its expression is normally restricted to the brain, but BC neoplastic cells that acquire the ability to synthesize it have an increased ability to cross the BBB and invade the brain parenchyma
[25]. In addition, β4 integrin signaling promotes tumor endothelial adhesion and extravasation by enhancing vascular endothelial growth factor (VEGF) expression, activated by hypoxia, which promotes vascular remodeling and increased permeability
[26][27][28][26,27,28]. After the BBB has been breached, reactive astrocytes activated by contact with cancer cells initiate an anti-tumoral response by secreting plasminogen activators
[29]. In the early phases of the anti-tumoral response, this promotes the activation of plasmin, which in turn is responsible for the elimination of neoplastic cells
[30]. However, some tumor cells can escape by producing anti-plasminogen activator serpins
[31]. In the later phases of metastasis growth, reactive astrocytes have a tumor-promoting effect through the creation of a microenvironment that favors metastasis progression
[32]. The summary of BC BM biological mechanisms is represented in
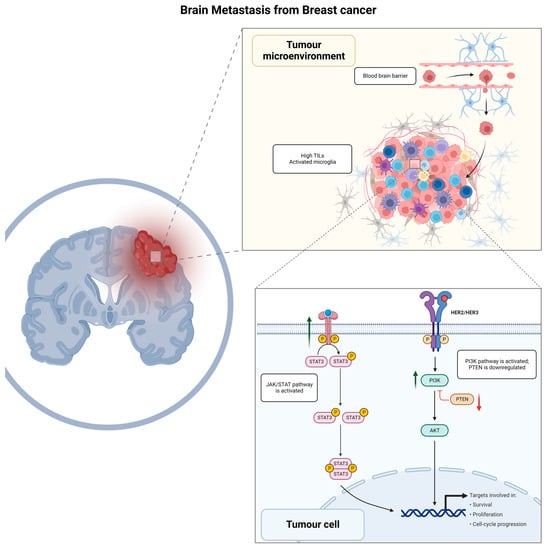
Figure 1.
Figure 1. Graphical summary of biological mechanisms involved in brain metastasis development for breast cancer patients. TILs, tumor infiltrating lymphocytes; JAK/STAT, Janus kinase signal transducer and activator of transcription; HER2/HER3, human epidermal growth factor receptor 2/human epidermal growth factor receptor 3; PI3K/AKT/PTEN, phosphoinositide 3—kinase-protein kinase B (Akt)—phosphatase and tensin homolog.
2.1. JAK/STAT Signaling Pathway
Reactive astrocytes following BM are characterized by activation of the JAK/STAT signaling pathway that promotes tissue repair and scar formation following brain injury
[33]. Astrocytes expressing pSTAT3 increase the recruitment of CD74+ microglia-macrophages, which in turn induces the activation of the macrophage migration inhibitory factor (MIF)
[34][35][34,35]. The activation of the CD74-MIF axis has an immunosuppressive effect on peritumoral microglia macrophages, creating a metastasis-favorable microenvironment
[36]. In addition, STAT3+ astrocytes have an immune suppressive effect by inducing the expression of programmed cell death–1 (PD-1) and programmed cell death–1 ligand 1 (PD-L1)
[37][38][39][40][41][37,38,39,40,41]. Furthermore, BC BM has been observed to be highly immunogenic, with high levels of tumor-infiltrating lymphocytes (TILs) frequently reported within the tumor and the surrounding stroma
[42][43][42,43].
2.2. Immune Checkpoints Mechanisms
A study of 233 patients with solid tumors of various origins and concurrent BM has demonstrated PD-L1 expression in 23.6% of BMs, wherein 18% (19 cases) showed PD-L1 expression in both the primary tumor and the BM (22C3 anti-PD-L1 antibody, Dako Agilent)
[44]. Interestingly, authors have also shown that IHC evaluation of CD8 (a co-receptor for the T-cell receptor) resulted in its expression being associated with higher PD-L1 expression, both in the primary tumor and BM, which confirms the ongoing lymphocytic reaction in the BM microenvironment
[44]. Significant correlations were identified between the infiltration of CD8-positive lymphocytes in primary tumors and the BM characteristics, with a higher incidence of multiple BMs observed in cases with lower levels of CD8 infiltration in the primary tumors.
[44][45][44,45]. Griguolo et al. have also confirmed that TNBC BMs showed a significantly higher percentage of intra-tumoral CD8+ cells and a higher density of CD163+ M2-polarized microglia/macrophages within the HER2-negative BC BMs, associating the latter with a worse prognosis, but identifying another potential therapeutic target to be explored
[46]. The authors emphasize that, paradoxically, in both TNBC BMs and HR+/HER2− BC BMs, the interaction between CD163+ microglia/macrophages and T lymphocytes was associated with a better outcome
[46]. A study by Noh et al. exploring the evolution of the tumor microenvironment in BC BM found that a lower CD8+ T cell count, low CD86+ M1 macrophage count, and high M2/M1 macrophage ratio in the BC BM compared to the primary tumor were related to unfavorable clinical outcomes
[47]. Furthermore, Giannoudis et al. have conducted an analysis of 55 samples consisting of 26 paired primary BCs and their BMs, assessing TILs and mRNA expression
[48]. Authors have demonstrated a significant reduction of TILs in BC BMs in comparison to primary BCs, with an 11.5% high-TILs count in primary tumors (>40% stromal TILs) versus only 3.8% in BC BMs
[48]. A total of 112 immune-related gene levels were found to decrease in BC BMs compared to primary BCs, including PD-L1 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) (false discovery rate < 0.01, log2 fold-change > 1.5), which are involved in cytokine–chemokine signaling, immune cells migration, matrix remodeling, and metastasis
[48]. CTLA-4 is one of the fundamental immunosuppressive cytokines, mainly activated on T-cells, which suppresses the immune response, and potentially prevents cancer cells from being attacked by the immune system. CTLA-4 genetic variants have been shown to play a role in BC progression, presenting a prognostic value
[49] and making CTLA-4 a potentially attractive target for BC immunotherapeutic approach development, wherein the evaluation of its mutations may become markers for genomics-based precision medicine and effective BC treatment
[50]. The depletion of T-cell response could be driven by
ARG2 (Arginase 2) upregulation, which was found in BC BMs and confirmed immunohistochemically (ARG2 protein expression was associated with worse breast–brain metastasis-free survival (
p = 0.027) and OS (
p = 0.019)), so ARG2 could be another potential marker of BC distant metastasis and a therapeutic target in BC BM, as proposed by authors
[50]. The model of mouse mammary carcinoma by Sham et al. has provided an analysis of EMT cell lines, wherein tumor cells acquired resistance to radiotherapy, changing their phenotype and causing them to acquire higher migratory and survival rates, which in turn represents a higher metastatic potential
[14]. The authors performed a next-generation sequencing (NGS) to explore underlying genes responsible for EMT cell culture and found upregulation of
PDL-1, AXL, GAS6, and
APCDD1, which are believed to contribute to radioresistance acquisition through the JAK/STAT/PI3K pathway
[14]. Based on this hypothesis, the authors determined the levels of PD-1 and CTLA-4 proteins expression, as they are known to be associated with the JAK/STAT/PI3K pathway
[51]. Indeed, these proteins were confirmed to be overexpressed in the EMT cell line by Western blot
[14]. The immune microenvironment has a profound influence on the outcome of patients with BMS from BC, with a significantly poorer prognosis in tumors with increased PD1/PDL1 expression
[46]. However, these patients could benefit from targeted immune therapy
[52][53][52,53].
2.3. PI3K-Akt Signaling Pathway
The activation of the phosphoinositide 3-kinase (PI3K)-protein kinase B (Akt) signaling pathway has been observed in a large proportion of BCs with BM
[54][55][54,55]. Activation of this pathway leads to a more aggressive phenotype of neoplastic cells, with increased survivability, proliferation, and angiogenetic potential
[56][57][56,57]. Patients with BMs harboring activation of the PI3K-Akt signaling pathway have a worse outcome compared to patients with the same-stage disease and lower PI3K-Akt activity
[54]. In addition, it contributes to the modulation of the peritumoral immune microenvironment through the activation of immunosuppressive regulators such as PD-1 and CTLA-4
[39]. Interestingly, a functional link has been reported between the PI3K and STAT3, where a synergistic interaction of the two pathways has been observed in murine neoplastic cells
[58]. This crosstalk is mediated by the cytoplasmic tyrosine-protein kinase BMX, a member of the TEC kinase family known to be a STAT3 phosphorylator
[59][60][59,60]. TEC kinases, including BMX, are in turn activated by PI3K, with a positive effect on STAT3 activation
[61]. This inter-dependency is further demonstrated by the fact that PI3K inhibition significantly reduces STAT3 phosphorylation and activation
[62].
Another important component activating the PI3K-AKT pathway is HER2-HER3 dimerization
[63], which plays a central role in the biology of BC with BM
[54][57][64][65][54,57,64,65]. Patients with HER2+ disease are at increased risk of developing BMs
[3][8][66][3,8,66], with HER2 overexpression often preserved at the metastatic site, and associated with the overexpression of HER3
[63], a coreceptor that forms heterodimers with HER2, thus playing a role in the neoplastic transformation of HER2-enriched BCs
[67]. This provides a further therapeutic perspective on patients with BMs from BC, as HER3 inhibitors are available and have been proven to be effective in increasing the sensitivity to PI3K inhibitors
[68].
2.4. PTEN
Another frequent occurrence in BC BM is the loss of phosphatase and tensin homolog (PTEN)
[69]. This event has been observed to be more frequent in BM compared to dissemination to other organs, suggesting a role of the local microenvironment in inducing this alteration
[70]. This mechanism has indeed been demonstrated to depend on epigenetic regulation through micro-RNAs (miRNAs) secretion by astrocytes
[71]. In addition, PTEN loss also induces the secretion of cytokine–chemokine ligand 2 (CCL2), which is responsible for the creation of a pro-inflammatory microenvironment
[72], and recruitment of ionized calcium-binding adapter molecule 1 (Iba1)+ myeloid cells that reduce apoptosis and promote growth in neoplastic cells
[71].
3. Targeting Therapies for Breast Cancer Patients with Brain Metastasis
According to the latest ASCO-SNO-ASTRO guidelines, surgery with subsequent radiotherapy on the operated field may be offered for patients with one brain mass without primary cancer diagnosis or for large tumors with mass effect. Local treatment with radiotherapy is reasonable for symptomatic BMs, regardless of the systemic therapy administration
[73][82]. There is not one recommended sequence for patients receiving both local treatments and systemic therapy
[74][83]. Medical treatments for BC are mainly based on hormone receptor and HER2 status, while some novel target therapies are available depending on the tumor molecular profile
[7][73][75][7,82,84]. Pharmacological approaches for patients harboring encephalic disease do not differ from patients who do not, except for HER2-positive BC, for which guidelines providing a specific treatment algorithm have been provided
[76][85].
3.1. HR+/HER2−—Breast Cancer
Local treatments (surgery and radiation therapy) remain the gold standard for BMs from HR-positive/HER2-negative (HR+/HER2−) BC
[73][74][82,83]. Existing practice guidelines for MBC treatment recommend sequential endocrine/targeted therapy until the exhaustion of available agents, before systemic cytotoxic chemotherapy administration
[74][77][78][83,86,87]. Although BMs in HR+/HER2− BC show a lower incidence compared to HER2+ and TNBC subtypes, endocrine therapy has been found to be beneficial for both CNS and systemic management
[74][79][83,88]. Indeed, tamoxifen and its metabolites have been reported to achieve effective concentrations in the CNS
[80][89]. First-line treatment for this population is based on endocrine therapy plus cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, namely palbociclib, ribociclib, or abemaciclib
[81][90]. These three agents are capable of crossing the BBB, but there is a lack of clinical data to inform a CNS-specific response rate. Indeed, no data on CNS outcomes are available for ribociclib and palbociclib
[74][83]. Regarding abemaciclib, a single-arm phase II clinical trial did not meet its primary endpoint of intracranial overall response rate in HR-positive BC with BMs, although the drug achieved an excellent concentration in cerebrospinal fluid. It is worth noting that palbociclib has been shown to be effective in BMs of different tumor types, including BC, and to harbor a CDK4/6 alteration
[82][91]. Companion endocrine therapy for CDK4/6 inhibitors could be represented by fulvestrant or letrozole. Elacestrant, an oral selective estrogen receptor degrader approved by the FDA, could be an option for patients harboring an
ESR1 mutation after CDK4/6 exposure
[83][92]. However, no data on CNS efficacy are available for this drug at the time of writing.
Furthermore, preclinical data have shown that the serine/threonine protein kinase AKT (protein kinase B) inhibitor capivasertib is active in combination with fulvestrant, providing the basis for future studies
[84][93]. Given the promising results of the phase II FAKTION trial (NCT01992952)
[85][86][94,95], capivasertib was further investigated in the phase III CAPItello-291 trial (NCT04305496), with results recently reported at the San Antonio Breast Cancer Symposium 2022
[87][96]. Although patients with BMs were included, results in this subgroup are pending.
Another crucial pathway in BC is the PI3K/Akt/mammalian target of rapamycin (mTOR) signaling, wherein activating
PIK3CA mutations, occurring in about 40% of hormone receptor HR+/HER2− MBCs, are driver events for tumorigenesis and tumor progression
[57]. This pathway has been successfully targeted by the α-selective PIK3CA inhibitor alpelisib
[55], with a manageable safety profile (mainly diarrhea and hyperglycemia). The incidence of BMs is particularly high among patients with HR+/HER2− BC patients carrying a
PIK3CA mutation
[88][97], and one report has shown a gain of
PIK3CA mutation in BM samples despite its absence in a primary BC
[89][98]. Real-world evidence suggests that alpelisib may have CNS activity
[90][99]. However, the low number of cases reported justifies the need for additional investigations to prospectively evaluate alpelisib efficacy in patients with BC BM
[91][100].
3.2. Triple-Negative Breast Cancer
Among the three main BC subtypes, TNBC is characterized by a limited therapeutic armamentarium
[92][101]. However, this scenario is rapidly expanding thanks to innovative options based on the characterization of individual tumor molecular profiles
[93][94][95][96][102,103,104,105]. At the moment, the treatment strategy for most TNBCs relies on chemotherapy, immunotherapy (if PD-L1 is positive), and target therapy with poly (adenosine diphosphate–ribose) polymerase inhibitors (PARPis) based on germline
BRCA1/2 (
gBRCA1/2) status.
[76][97][98][99][85,106,107,108]. In patients with MBC harboring a
gBRCA1/2 pathogenetic variant, the two PARPis, talazoparib and olaparib, showed a benefit in survival outcomes
[100][109]. Regarding BMs, a higher frequency of BMs is reported in this population. In the EMBRACA trial (NCT01945775), talazoparib resulted in significantly longer progression-free survival than standard-of-care chemotherapy; this was also found in the subgroup of patients with CNS metastases
[101][110]. However, the final overall survival analysis was not statistically significant
[102][111]. It is worth noting that the enrolled population comprised both TNBC and HR+ BC; no specific analysis for BMs in each subgroup is available, given the limited number of patients with BMs enrolled in the study (about 15%). The same concern could be raised for the OlympiAD trial, although in this study no specific subgroup analysis for BMs is available
[103][112]. In these studies, PARPis have shown mainly hematological and gastrointestinal toxicity.
On the other hand, immunotherapy has demonstrated potential intracranial efficacy among patients with MBC
[104][105][113,114]. Indeed, recent studies demonstrated that TNBCs exhibit higher PD-L1 expression than other BC subtypes, which is associated with remarkable genomic instability and higher immune infiltration
[106][115]. The role of the immune microenvironment and PD-L1 expression in the brain and metastatic brain tumors is poorly understood
[46][48][46,48]. Chehade and colleagues have studied 59 immunotherapy-naïve BC patients with BMs in a single-center retrospective cohort study, wherein 15.3% had PD-L1+ BM, with the highest proportion (25%) among those with TNBC (SP142 antibody, Ventana)
[52].
TIn this study, the concordance in PD-L1 expression between primary BC and BM in TNBC specifically could not be studied due to small sample sizes, but authors emphasize that PD-L1 expression has previously been reported to be higher in the primary tumor compared to metastatic sites (63.7% vs. 42.2%,
p < 0.0001), specifically in TNBC
[52][107][52,116]. Hence, it was proposed that PD-L1 staining should be performed both on the primary and metastatic tumor to maximize the opportunity for ICI therapy administration
[107][116].
Focusing on clinical data, the combination of chemotherapy and pembrolizumab improved overall survival with manageable safety in patients with PD-L1+ (CPS ≥ 10) advanced TNBC in the phase III KEYNOTE-355b study
[108][117].
OIn this study, only a few patients with BMs were enrolled; thus, it is not possible to derive any conclusion
[109][110][118,119]. The IMPASSION-130 trial (NCT03483012) has demonstrated a benefit of atezolizumab in combination with nab-paclitaxel for TNBC treatment; however, no progression-free survival (PFS) benefit for BM patients has been observed, although this population had a relatively small representation (6.3%)
[111][120].