1. ROS Production by 5-ALA-PDT
ROS are reactive oxygen species that contain unpaired, highly unstable electrons. They include the superoxide anion radical (O
2−), the hydroxyl radical (OH
−), and nonradical oxidants, such as hydrogen peroxide (H
2O
2) and singlet oxygen (
1O
2)
[1][2][30,31]. ROS are involved in a number of physiological functions, such as cell signaling, cell differentiation, sensing of oxygenated cells, adaptive immunity, elimination of unwanted mitotic cells, and mitochondrial physiology
[3][4][5][32,33,34]. Mitochondria and NADPH oxidases are responsible for ROS production. ROS are produced by complex I and III of the electron transport chain, which remove an electron from amino acids, glucose, and lipids, transferring it to O
2 [6][35]. Complex III releases O
2− and H
2O
2 into both the intermembrane space (∼80%) and the mitochondrial matrix ∼20
[7][36]. Most of the O
2 released in the mitochondrial matrix is dismantled into H
2O
2 by manganese superoxide dismutase (MnSOD or SOD2). SODs use metal ions, such as copper, iron, manganese, and zinc as cofactors. There is also SOD1(Cu/ZnSOD) in the cytosol and SOD3 (Cu/ZnSOD) in the extracellular matrix
[8][37]. H
2O
2 leaves the mitochondrial membrane through specific proteins of the aquaporin family
[9][38].
H
2O
2, a major signaling molecule involved in cancer, can damage DNA, proteins, and lipids after undergoing Fenton chemistry with Fe
2+ to form OH
−. It can be reduced and converted to H
2O and O
2 by peroxiredoxins (PRX), glutathione peroxidase (GPX), and catalase
[10][39]. ROS are also produced by leukocytes in response to B- and T-cell inflammatory mediators through the transmembrane proteins NADPH oxidase (NOX)
[11][40]. NOXs transfer electrons across biological membranes to produce O
2− and H
2O
2, which can diffuse across the membrane. The release of growth factors activates NOX-generated ROS that act as secondary signaling molecules, inactivating peroxiredoxin 1 (PRX1), ensuring the continued accumulation of H
2O
2 [12][41]. NOX-derived ROS stimulate cell survival, genomic instability, metastasis, invasion, and angiogenesis in many common cancers
[13][42]. There is a delicate balance between the amount of ROS and antioxidants; both high levels of ROS (oxidative stress) and excessively low levels of ROS (reductive stress) are deleterious
[14][43].
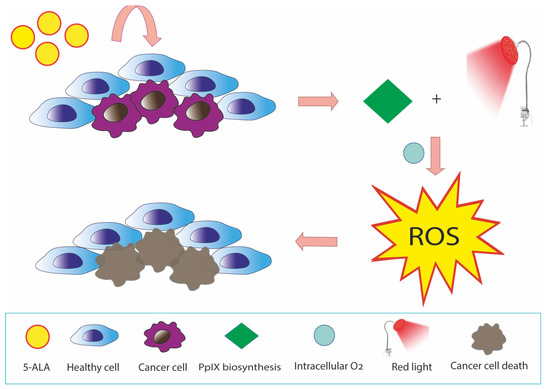
5-ALA-PDT is a promising anticancer therapy with ROS generation by PpIX in mitochondria.
[15][44] (
Figure 13). Cellular uptake of exogenous ALA into the cytosol occurs through peptide transporter 1 (PEPT1), proton-coupled amino acid 1 transporter (PAT1), taurine transporter (TauT), and GABA transporter 2 (GAT2)
[16][45]. The 5-ALA-induced ROS burst resulted in a loss of MMP, ATP production, and mitochondria-dependent apoptosis through upregulation of BAX and downregulation of BCL-2/BcL-xL. In turn, the decrease in MMP and ATP synthesis stimulates ROS production
[17][46]. It can enhance cytochrome c oxidase (COX) activity of the mitochondrial respiratory chain, which can thus disrupt the Warburg effect, generate intracellular ROS, and induce caspase-dependent apoptosis in cancer cells
[18][19][20][19,20,47].
Figure 13. ROS production induced by 5-ALA-PDT in cancer cells. 5-ALA is a pro-photosensitizing endogenous metabolite converted in PpIX in the heme biosynthetic pathway. PpIX accumulation in cancer cells is commonly higher than that in normal cells, as cancer cells are deficient in some enzymes of the pathway. In cancer cells, the presence of molecular oxygen and the excitation of PpIX under irradiation produces ROS that cause DNA and cell membrane injury through lipid peroxidation. This reaction results in damage to mitochondria and other cellular organelles and finally cellular death.
ALA increases heme biosynthesis by increasing heme protein. Through iron ion insertion, ALA converts to protoporphyrin IX, generating heme
[21][48]. ALA-PDT could play a tumor suppressing role in precancerous and oral cancer cells through the ROS/MMPs pathway or by stimulating cells to release TGF-β. However, the potential relationship between TGF-β and ROS in human dysplastic oral keratinocytes cells treated with 5-ALA-PDT remains unknown
[22][49]. Excessive ROS production causes cell death; in fact, singlet oxygen from 5-ALA-PDT can induce necroptosis via RIP-3 in glioblastoma cells
[23][50]. The antitumor action of 5-ALA-PDT is also manifested by stimulating antitumor immunity through activation of heat-shock protein and inhibition of heme oxygenase-1 (HO-1) by hyperthermia and by acting as a radiosensitizer, increasing the delayed production of ROS in the cytoplasm after ionizing irradiation (IR)
[24][25][51,52]. The combination of IR and 5-ALA may help overcome hypoxia-induced RT resistance in prostate cancer. 5-ALA can promote the IR-induced Fenton reaction by forming superoxide and causing ferroptosis in prostate and esophageal cancer cells
[26][27][53,54].
ROS generation by 5-ALA-PDT in human cholangiocarcinoma cells was dependent on both incubation time and concentration. The level of ROS gradually increased to 0.5 mM after irradiation as PpIX synthesis after 24 h incubation was saturated above 0.5 mM of 5-ALA
[28][55]. The combination of 5-ALA and ferrous sodium citrate (SFC) resulted in the selective accumulation of PS PpIX and reduced cell viability in a concentration-dependent manner. 5-ALA-SFC enhanced ROS generation and reduced cell viability of the human gastric cancer line MKN45
[29][56].
2. 5-ALA-PDT Treatment in Different Type of Cancers
2.1. In Vitro Studies
2.1.1. Colorectal Cancer
Colorectal cancer (CRC) has been reported as the second leading cause of tumor deaths worldwide. One of the dangerous facts associated with colon cancer is the recurrence of tumor cells from the residual cells, leading to metastasis and carcinoma. Although various advancements have been made in cancer therapy, there are still two critical factors that are major threats to human life. They include recurrence of colorectal cancer and metastatic spread of cancer. Depending on the stage of cancer, 25% of patients have metastasis while 50% of patients develop metastasis after their follow up treatment. Therefore, there was a strong need to come up with a treatment option other than conventional radiotherapy or chemotherapy treatment options that can effectively treat tumors at the initial stage with its photochemical property.
PDT is one of the best nonconventional treatment options for CRC, playing a major role in the progression of immune response that helps in the defense mechanism against cancer. In CRC, the rectum opening is commonly used to access the colorectal cancer site. Colonoscopy endoscopes are used to administer the PS and specific wavelength to the target area to activate PS drug.
A study on CRC cell lines SW480 and SW460 evaluated the effect of 5-ALA-PDT treatment on interleukin secretion. In CRC, there is an increased production of interleukins IL-6, IL-8, and IL-10, which cause tumor growth, cell proliferation, metastasis, and angiogenesis
[30][57]. Cell lines were incubated with 1000 μM of 5-ALA for 4 h. Cell viability, evaluated by MTT assay, was decreased in cancer cell lines SW480 and SW460 after 5-ALA-PDT treatment, as compared to the control group, decreasing the concentration of interleukins IL-6 and IL-10. 5-ALA-PDT showed no effect on IL-8 secretion.
2.1.2. Glioma
Among different brain cancers, malignant gliomas are very common and account for nearly half of brain tumors. Conventional treatment methods of human glioma consist of surgery, chemotherapy, and radiotherapy, which sometimes have serious consequences. Due to the cancer’s recurrence, surgery is not a viable option and due to the development of resistance and other serious side effects, chemotherapy and radiotherapy are only partially successful. In a study conducted on human glioma cells, intracellular levels of PpIX after 5-ALA treatment of human glioma cells were evaluated. Rat astrocytes and human glioma cells were pretreated with arsenic trioxide (ATO). The results showed that 5-ALA-PDT showed an increased accumulation of PpIX followed by pretreatment with ATO relative to the control groups. Apoptosis activity and viability of the cells in the glioma cells pretreated with ATO compared to control groups were increased and decreased, respectively. It was also observed that the mRNA expression of the CPOX enzyme in Porphyrin synthesis was increased after pretreatment with 0.1 μM ATO. Thus, 5-ALA-PDT pretreatment with ATO can prove to be an effective strategy to improve 5-ALA-induced FGR and PDT in glioma
[31][58].
2.1.3. Breast Cancer
The effect of 5-ALA-PDT along with light irradiation on adenocarcinoma breast cancer cell lines MCF-7 and hepatocellular carcinoma cell lines HepG2 was studied. 5-ALA-PDT and light irradiation alone were not able to induce cytotoxic effects and DNA damage in the cell line, but when used together with a suitably high concentration, PDT was successful. 5-ALA-PDT promoted cell death in a concentration-dependent manner. Furthermore, 5-ALA-PDT created significant amounts of micronuclei in the MCF-7 and HepG2 cell lines, as seen in binucleated cells. Additionally, cancer cell lines treated with 5-ALA-PDT exhibited significantly greater mean percentages of DNA damage and tail moment ratio compared to untreated cells, cells treated with 5-ALA or laser irradiation separately, or cells that had not been treated at all. In comparison to untreated cells or cells treated with 5-ALA or laser irradiation alone, MCF-7 and HepG2 cells treated with 5-ALA for 4 h and then exposed to laser irradiation for 4 min and PDT enhanced the percentages of cell death in a highly significant way. After increasing the concentration of 5-ALA from 0.5 mM to 2 mM for MCF-7 cells, the treatment decreased cell viability from 75% to 46%, compared to 88% for non-treated cells. Additionally, by increasing the concentration of 5-ALA in HepG2 cells, the percentage of cell viability was substantially decreased from 86% in untreated cells to 76% at 0.5 mM 5-ALA-PDT and 63% at 2 mM 5-ALA-PDT. Micronuclei were induced and significantly increased in both cancer cell lines at concentrations of 5-ALA of 0.5 mM and 1 mM and PDT treatment. In the comet assay analysis, DNA damage in the MCF-7 and HEPG2 cell lines were measured post-5-ALA-PDT and laser irradiation. DNA damage was measured by tail DNA for both cells and it was seen that tail DNA content increased in both MCF-7 and HEPG2 cell lines as compared to untreated cells
[32][59].
2.1.4. Bladder Cancer
The effect of 5-ALA-PDT combined with tumor-necrosis-factor-related apoptosis inducing ligand (TRAIL) was studied on bladder cancer cell lines
[33][62]. The individual and combined effect of these drugs were studied on three bladder cancer cell lines: SW780, 647V, and T24. These cell lines were treated with 5–100 ng/mL TRAIL for 18 h and the cytotoxic effect of TRAIL and 5-ALA-PDT was observed in all these cell lines. SW780 showed a sensitive nature to TRAIL, while 647 V and T24 showed resistance to TRAIL. Cytotoxic concentrations of TRAIL from 5–50 ng/mL were effective against all bladder cancer cell lines, while an increase in concentration had no effect on the cytotoxic nature of the cell lines. In the next step, bladder cancer cell lines were pretreated with low-dose 5-ALA (5–50 μM) for 4 h and with visible light (400–750 nm, 7.5 J/cm
2). The photocytotoxicity of 5-ALA with visible light resulted in decreased cell proliferation. Then, cell lines were incubated with TRAIL for 18 h. At the end, the combined effect of pretreated 5-ALA-PDT and TRAIL were observed on bladder cancer cell lines. Bladder cancer cell lines were pretreated with low-dose 5-ALA-PDT (25 μM) and visible light (400–750 nm, 7.5 J/cm
2) for 3 h, and cell lines were then incubated with TRAIL for 18 h. The therapy of 5-ALA-PDT along with TRAIL induced cell death in all bladder cancer cell lines and these results showed the efficacy of a combined therapy of 5-ALA-PDT and TRAIL for the treatment of bladder cancer.
2.1.5. Esophageal Cancer
5-ALA-PDT treatment along with specific tyrosine kinase inhibitors TKIs were investigated on esophageal carcinoma cell lines ECA-109
[34][63]. In this study, ECA-109 cells were incubated with a medium containing EGFR tyrphostin AG1478 or PI3K inhibitor LY294002 (small synthetic compound molecules with specific inhibition of EGFR activation) and then with 5-ALA, and the cells were irradiated with the laser 6 h later. The cell viability was measured with the MTT assay, and the migration ability was detected 24 h post-5-ALA-PDT.
PDT can stimulate a variety of signaling pathways in tumor cells. Esophageal carcinoma shows a high EGFR expression, so it was thought that an appropriate therapy is the use of EGFR inhibitors. PI3K/AKT is an important downstream signaling pathway. The study found out that TKIs blocked the pi3k/AKT and PKB/ AKT signaling pathways, blocked EGFR over expression, inhibited cell proliferation, and hampered tumor growth. The migration and proliferation ability of cell lines were measured by the MTT assay, which revealed that 5-ALA-PDT treatment reduced the cell proliferation and migration of cancer cells. Intriguingly, as the concentration of 5-ALA-PDT treatment continued to increase, a gradual decrease was seen in the concentration of inhibiting cells. Thus, the combination therapy with 5-ALA-PDT and EGFR inhibitors has a synergistic effect on decreasing the proliferation and migration of ECA-109 cells.
2.1.6. Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma
PDT, using the topical administration of 5-ALA, can serve as a promising anti-tumor method in the treatment of oral potentially malignant disorders (OPMDs) and oral squamous cell carcinoma (OSCC). The study of Wang shows the development of a high-adhesion-strength dry polyacrylic acid (PAA)-chitosan (CHI)-ALA interpenetrating network hydrogel (PACA) patch that can be applied topically to a moist surface and can easily work as a drug delivery model for 5-ALA
[35][64]. Hydrogels, due to their biocompatibility and adhesion to biotic surface, are currently used in research. The author studied the effect and compared the photo cytotoxicity of 5-ALA and PACA hydrogel adhesives in three dysplastic oral keratinocyte cells: DOK; OSCC cells CAL-27; and normal oral keratinocyte cells HOK. Studies have confirmed that 5-ALA works best when applied topically. Cells were incubated with 5-ALA and PACA hydrogels at a concentration of (20–100 μg/mL). No effect of cell death was observed in cell lines treated with PACA hydrogels. When the cells were treated with light irradiation, prior to PDT treatment, ALA and PDT inhibited the growth of CAL 27 and DOK. DOK cells showed a reduction in cell viability at a concentration of 60–100 μg/mL. Cell viability in DOK decreased with increasing concentration at 100 μg/mL. CAL 27 cells were more susceptible to PDT treatment than DOK cells. Cell viability was decreased more in CAL 27 and DOK cells than HOK cell lines. Cell viability in HOK cells were decreased to a much lesser extent. The study revealed that PACA-PDT induces apoptosis in DOK cells in vitro. DOK cells were treated with 100 μg/mL PACA hydrogels and illuminated with a 653 nm laser light. Results were checked by flow cytometry. DOK cells were treated with PACA-PDT and then 5-ALA-PDT. Results showed that PACA hydrogels without PDT could not induce apoptosis in cells. PDT irradiation was mandatory for the apoptosis to happen in a cell. The proportion of apoptosis was much higher in the PACA-PDT group than in the 5 ALA-PDT group alone. In conclusion, the authors created a new dry mucoadhesive hydrogel-loaded ALA to provide increased PDT for OPMDs. The results showed that the PACA hydrogel has excellent biocompatibility, wet adhesion qualities, and sustained ALA diffusion, indicating that it may be used as an oral mucosal medication delivery. PACA hydrogel-mediated PDT demonstrated strong anticancer activity in OPMDs under exposure to 635 nm laser irradiation in vitro. Dry hydrogel patches would also offer a convenient substitute for conventional approaches for the clinical treatment of OPMDs. These patches have a high comfort and acceptability
[35][64].
2.1.7. Skin Cancer
The main aim of the study by Chi et al. was to check the effectivity of 5-ALA combined with gold nanoparticles on cutaneous squamous cell carcinoma (CSCC)
[36][65]. It is one of the most dangerous cancers and the only possible and standard treatment for localized CSCC is surgical excision, cryosurgery, and desiccation, but due to numerous side effects of these treatments, they are not preferred. Among the non-surgical options, PDT is preferred because of better cosmetic outcomes, low morbidity, and is considerably less harmful than chemotherapy and radiotherapy. 5-ALA-PDT treatment alone in clinical settings is shown to have good results but when combined with some other agents, its effectiveness increases to a higher level. For this purpose, gold nanoparticles (GNP) were designed and used with 5-ALA-PDT treatment.
Skin cancer with metastatic melanoma (MM) is deadly. In the study by Naidoo et al., the authors developed a PS multicomponent nanoparticle drug conjugate carrier system that specifically targets MM cells via biomarkers in order to enhance MM PDT
[37][66]. An antibody-metalated phthalocyanine-polyethylene glycol-gold nanoparticle therapeutic combination was successfully synthesized and characterized. In vitro-grown MM were used in experiments with PS active medications that target PDT at 673 nm. Results revealed that this medication combination boosted PDT for MM by significantly improving cytotoxic and late apoptotic cellular death in cells as well as PS subcellular localization. The ability to cross biological barriers, ease of functionalization, and ability to trigger photothermal cell death due to their metalated composition are all reasons why gold nanoparticles (AuNPs) can be employed as drug carriers in PDT applications to improve PS passive uptake in tumor cells. A highly sensitive and specific biomarker for MM drug uptake targeting, melanoma inhibitory activity (MIA), is an antigen that is particularly overexpressed on melanoma cells alone. To increase medication solubility and active MM tumor targeting uptake, it would, therefore, seem to be highly ideal to conjugate an MM tumor-targeted antibody, such as anti-MIA, onto a sulpho pure ZnPcS PS carrying AuNPs surface. ZnPcS4 PS medicine was attached onto the surface of amine functionalized AuNPs that also contained Anti-MIA antibodies linked to their surface to actively optimize PS medication administration and increase its uptake and absorption within MM target cancer cells. The outcomes of this study clearly enhanced PDT treatment for this type of skin cancer. These findings suggest that ZnPcS4 is an effective PS for PDT on MM due to its ability to significantly induce cell death. However, the IC50 of 2.5 M ZnPcS4 PS with laser irradiation applied was selected to determine whether the PS, when administered in a drug carrying conjugate, was capable of targeted and improved PDT, as it reported a significant decrease of 38% in cell viability and 48% in cellular cytotoxicity. The results of this work reveal that, in contrast to ZnPcS4 PS drug administration alone, the conjugation of anti-MIA Ab to ZnPcS4-AuNP-PEG5000-SH-NH2 inside the final PS drug conjugate actively and specifically improved ZnPcS4 PS drug uptake in MM cells. Thus, in comparison to control groups, the final PS drug combination saw dramatically increased PDT-induced cytotoxic cell death in MM cancer cells. These outcomes were also linked to AuNPs’ capacity for PDT-induced photothermal cellular death. In addition, compared to ZnPcS4 PS medication administration alone, it was discovered that the combination with PDT was found to have enhanced efficacy for most MM cells.
2.2. Clinical Applications of 5-ALA-PDT-In Vivo Studies
2.2.1. Esophageal Cancer and Barrett’s Esophagus
Esophageal cancer is often diagnosed at a later stage of metastasis and thus people are left with no options for surgery, chemotherapy, or radiotherapy. Thus, a safer option of PDT treatment can be applied to have effective results. PDT was approved by US Food and Drug Authority in 1996 for the treatment of esophageal cancer
[38][39][67,68]. Due to its high tumor specificity and less complications, it is widely used in research and clinical practices.
PDT using first generation PS Photofrin and Lazerphyrin has been adopted widely, but due to its complex composition, poor tissue penetration (Photofrin), and high incidence of hypersensitivity, it no longer possible to use it. Second generation photosensitizing agents, such as 5-ALA, known for their lower toxicity, faster metabolism, and lower phototoxicity, were then introduced.
2.2.2. Gastric Cancer
When it comes to assessing the size of the tumor and spotting metastatic lesions in gastric cancer, 5-ALA-photodynamic diagnosis (5-ALA-PDD) is a promising and secure diagnostic technique. PDD is a diagnostic method that involves the emission of light-induced excitation fluorescence to enhance early detection, without tumor destruction, after PS exposure to blue light. PDT may also have benefits in terms of reducing the invasiveness of the operation. To confirm the efficacy of the 5-ALA-mediated fluorescence technique for gastric cancer, additional research is required, including a prospective randomized controlled trial. Exogenously supplied 5-ALA is taken up by cells and utilized to create PpIX that exhibits red fluorescence when activated by irradiation of a particular wavelength, primarily visible blue light between 375 and 475 nm. This feature can be used to precisely detect cancer cells since they collect PpIX. PDD is a relatively novel method based on the tumor-specific accumulation of 5-ALA-induced PpIX
[40][71].
In terms of the usage of 5-ALA-PDD in the treatment of gastric cancer, it might be applied as a tool for assessing surgical resection margins and consequently support pathological diagnosis during surgery. Occasionally, judgements must be taken regarding how to proceed with surgery to treat stomach cancer, such as establishing the extent of the disease in patients with hazy margins. In these situations, 5-ALA-PDD can offer helpful data to assess the margins that would be enough for resecting the tumor. The most common type of distant metastasis and post-surgical recurrence in advanced gastric cancer with serosa-invading tumors is peritoneal metastasis from a primary gastric cancer, which is an incurable condition with a dismal prognosis. Since proper staging of gastric cancer, and the identification of peritoneal spread is a requirement for choosing the most suitable therapy, numerous attempts using novel techniques have been performed. Although staging laparoscopy is widely used in the care of patients with advanced gastric cancer to avoid needless laparotomy, it has limitations in terms of visualizing the cancer nest’s spread.
In patients with gastric cancer, staging laparoscopy with 5-ALA-PDD increases the diagnostic precision for peritoneal metastases and is safe. To compare the detection sensitivity of 5-ALA-PDD with that achieved using conventional white light, Kishi et al. studied the utility of 5-ALA-PDD with staging laparoscopy in patients with advanced gastric cancer that had invaded the serosa
[41][72]. In a mouse model of peritoneal metastases, which involved eight mice with 729 peritoneal nodes, they showed that the tumor detection rate of 72% using 5-ALA-induced fluorescence was much higher than that attained using white light (39%). In addition, in 13 patients undergoing staging laparoscopy, 3 metastatic lesions that were undetectable under white light were found with 5-ALA-induced fluorescence. They also compared the outcomes of the 5-ALA-PDD with those of the peritoneal fluid cytology and molecular diagnostic testing in 52 patients.
2.2.3. Head and Neck Cancer
Since head and neck anatomy typically permits simple laser access, early superficial lesions in the larynx, throat, and oral cavity constitute suitable PDT targets. The depth of the laser light’s penetration varies depending on its wavelength. In a summary of findings of the literature, the review from Biel et al. reported an 89% full response rate in patients with carcinoma in situ or early-stage head and neck tumors treated with PDT and Photofrin as a PS
[42][73]. According to preclinical results of Photofrin for head and neck cancer, two-part fractionated light treatment for tumor management may be advantageous. PDT offers patients a functionally appealing alternative.
A study carried out by Ahn et al. presented the experience treating patients with high-risk premalignant and microinvasive head and neck carcinomas with 5-ALA-PDT
[43][74]. The trial used noninvasive optical technologies to check the physiologic and photosensitizing qualities of the tissue to comprehend the relationship between lesion physiology and PDT effectiveness. However, considerably greater levels of oxygenation and blood volume were linked to higher rates of response to PDT in patients with tongue/floor of mouth lesions as well as those who had intact disease at the time of PDT, even though the study did not identify a correlation between blood volume and 3-month complete response. The authors found a relation between poor lesion oxygenation and local recurrences but found no association between lesion oxygenation and marginal recurrence. This phase one study was the first clinical study confirming an association between tumor hypoxia with higher rates of recurrence after PDT, and it lays the foundations for an individualized PDT based on the oxygenation characteristics of the lesions.
Some researchers carried out a study in which 5-ALA was administered orally as a dose of 60 mg/kg diluted in 50 mL water, 4–6 h prior to light delivery. Active signs were checked before, immediately after 5-ALA administration, every 15 min for the first two hours, and then hourly till the surgery
[44][75]. Utilizing a Ceralas Series GaAlAs diode laser, activating light was applied 4–6 h after 5-ALA administration. Participants in the study were illuminated to red light at total fluences of 50, 100, 150, and 200 J/cm
2 (629–635 nm). All patients received post-treatment instructions to stay out of the sun for three days. The Cooperative Group Common Toxicity Criteria were used to grade toxicity endpoints (CTCAE v.3.0). Patients were monitored weekly for the first three weeks, then every two weeks for the next three months, and then every year after that. Grade 3 toxicity by 30 days following PDT injection was used to spot dose-limiting toxicity (DLT). If there was a recurrence, patients were taken off protocol; however, if there was additional progression, they continued off protocol
[44][75]. A phase one series suggested that PDT is generally well tolerated in the treatment of head and neck malignancies in the premalignant and early stages. The frequency of side effects was anticipated, having an impact on discomfort and mucositis. The patient population did not achieve DLT. Two individuals were ultimately unable to get treatment with ALA-PDT due to Levulan’s influence on triggering hypotensive episodes and/or transaminitis; this led us to carefully choose patients with limited cardiovascular history before proposing PDT with ALA.
Local recurrence patterns initially looked more important among groups with a lower dose than the groups under increasing PDT doses. Local recurrences happened after PDT with rates of 57%, 33%, 25%, and 25% at 50, 100, 150, and 200 J/cm2, respectively. These results were complicated by a shorter follow-up for higher-dosage groups; the median follow-up for the lower light dose group was 55 months, but the median follow-up for the higher light dose group was 30 months, with the last three patients who were under observation being followed for 6 months. It is important to highlight those local recurrences that happened between 1.3 and 19.0 months (median) following PDT treatment.
2.2.4. Oral Cancer
Tongue cancer (under the category of oral cancer) is one of the most common malignancies in the oral region. Conventional therapies of surgery and chemotherapy have certain limitations due to new advancements in the therapies which have been introduced. One method is the use of 5-ALA–PDT treatment.
This study by Ogasawara et al. examined PpIX fluorescence in tongue tumor tissue to determine the best way to administer 5-ALA
[45][76]. The effect intraperitoneal, oral, and topical administration of 5-ALA-induced PpIX was observed in mouse-transplanted tongue cancer and in normal mice.
In the intraperitoneal mode, 5-ALA in the powdered form was dissolved in 0.2 mL saline and administered at concentration of 250–500 mg kg−1 while in oral administration it was given in the same concentration and given by means of a gastric tube. In the case of topical administration, an oil-to-water emulsion was made in which 20% 5-ALA was used. Two derivatives of 5-ALA esters, methyl ester and pentyl ester, were also used and these were compared to the topical application. 5-ALA was administered at time intervals of 1, 2, 3, 4, and 5 h and mice were killed prior to taking samples of the tongue. Fluorescence PpIX emission spectra (10-μm thick) were obtained using a spectrophotometer on a total of five successive frozen slices. The fluorescence microscopic image of the study revealed that red fluorescent light was distributed evenly in tongue tumor tissue 5 h after 5-ALA was administered. There was a weak PpIX accumulation in normal tongue muscles. The tumor group showed higher concentration of 5-ALA-induced PpIX after i.p. and p.o. administration. The control group had lower concentrations of PpIX than the tumor group, which had twice the concentration of PpIX. Maximum PpIX accumulation intensity was seen after 5 h p.o. administration of 5-ALA. Moreover, minimal PpIX accumulation in the tumor was also seen after topical administration of 20% 5-ALA ester derivatives cream. which was like the result of topical administration of 20% 5-ALA cream. Maximum PpIX accumulation in the tongue tumor tissue was seen at 5 h after oral administration of 5-ALA. This study on 5-ALA-PDT on oral cancer suggested that oral administration was the most effective treatment. On the contrary, the topical administration of 20% 5-ALA cream was associated with the lowest PpIX accumulation.
2.2.5. Breast Cancer
Breast cancer is one of the leading cancers in women. One out of eight women are affected by breast cancer. Although many therapies have been suggested for the treatment of breast cancers, i.e., hormonal therapies, surgical mastectomy, and combination therapies, there is still a need to improve treatment options for breast cancer to have more options available to patients. PDT, because of its antitumor effects, advanced technology, and effectiveness, is the latest technology used in clinical practices. A study conducted by Banerjee studied the effect of PDT in primary breast cancer. The 4T1 murine breast carcinoma and 2H11 murine endothelial cells lines were used as an experimental tumor model
[46][77]. Cytotoxicity, vascular epithelial growth factor expression, and apoptosis level and cell migration were checked. An increased cytotoxicity level was observed in 4t1 cell lines, whereas no significant difference in apoptosis level was observed in 2h11 cell lines. Cell viability was reduced to 60% after 5-ALA-PDT and thalidomide (TMD) treatment as compared to the control group that showed 100% viability. 5-ALA-PDT along with TMD treatment were used on the 2h11 cell line. TMD therapy decreased the VEGF expression in 2h11 cell lines. Migration ability of cells was evaluated through the wound healing assay. The motility inhibition of cells with 5-ALA-PDT with TMD treatment was increased from 20% to 24%. Combination therapy of 5-ALA-PDT with TMD increased the apoptotic activity and decreased VEGF expression, which in turn increased the effectiveness of PDT treatment
[46][77].
2.2.6. Brain Cancer
The effectiveness and safety of PDT administration to both healthy and cancerous brain tissue have been assessed in animal and human studies
[47][78]. Another study utilized an experimental orthotopic rat glioma model and reported the anti-tumor activity of 5-ALA-PDT
[33][62]. In another study, U-105MG glioma and CH-157MN meningioma cells were treated for 24 h with 5-ALA and then exposed to 12.4 mW/cm
2, 11 J/cm
2 light. Cell viability was measured after 24–48 h from 5-ALA-PDT treatment. Increased cytotoxicity was evaluated to glioma cells and attributed to the preferential accumulation of PpIX within glioma compared to meningioma cells. Repetitive 5-ALA-PDT treatments spaced out over long periods of time (weekly for up to 3 weeks) were found to significantly suppress high-grade gliomas (HGG) spheroid cell development. BT4C HGG tumors were orthotopically implanted in BD-IX rats to test these findings in vivo. At 4–5 h before PDT, 5-ALA (125 or 250 mg/kg) was given intraperitoneally (IP) 3 days after tumor cell implantation. Through the burr hole, PDT was carried out intravenously at the same depth as tumor cell implantation. Light at 632 nm was provided for 10–30 min at optical output levels of 7.5–30 mW from a 400 m flat cut fiber (4.5–54 J). The rats who had several (i.e., two or three) PDT sessions per week had considerably longer median survival times.
A human HGG spheroid model has also shown promising results for 5-ALA-PDT
[48][79]. In this experiment, 5-ALA was added to human HGG spheroids for 4 h before either 25 or 50 J/cm
2 of light was administered. More cell death occurred within the spheroid when low fluence rates to the same total fluence were used, which may be due to low fluence rates’ improved ability to store oxygen during PDT and continue the continuing creation of ROS by the photochemical process.
The most recent effectiveness data using a preclinical 5-ALA-PDT rat model were published
[49][80]. Athymic fox1 rnu/rnu male rats were orthotopically injected with human U87MG GBM cells. Five hours later, PDT was given. 5-ALA (100 mg/kg) was given Intraperitoneal 14 days after tumor cell implantation. Under the supervision of an MRI, a 350 m flat cut quartz fiber was implanted into the tumor and used to provide light (635 nm) from a diode laser. The interstitial 5-ALA-PDT at 30 mW-treated animals showed symptoms of increased intracranial pressure (ICP), which was lethal in almost 60% of the animals. In the 4.8 mW group, no fatal or serious side effects were noticed. Although the per-group sample sizes were rather modest, the results revealed that fractionated PDT was superior to a single PDT session because significant tumor necrosis was generated in both the low and high fluence rate groups.
In comparison to spheroids treated with a single PDT session, those treated with numerous PDT sessions exhibited much less development potential. These results serve to emphasize the importance of continuing research on prolonged light delivery for PDT in brain cancer.
Additionally, 5-ALA-PDT’s in vitro cytotoxicity has been contrasted with that of 5-ALA derivatives, such as methyl-ALA (m-ALA), hexyl-ALA (h-ALA), and benzyl-ALA (b-ALA). Various doses of 5-ALA and ALA derivatives (0.025–5.0 mM) were treated with human HGG spheroids for 4 h. After 635 nm light (25 J/cm2, 25 mW/cm), 5-ALA and m-ALA (0.05 mM) had comparable cytotoxic effects. However, under the same circumstances, h-ALA and b-ALA produced more cytotoxicity. Further analysis of 5-ALA and h-ALA revealed that, at concentrations 10–20 times lower than those of 5-ALA, PDT with h-ALA produced a cytotoxic response comparable to 5-ALA-induced PDT.
5-ALA-PDT treatment has demonstrated efficacy as a therapeutic option against human glioma. Based on these results, 5-ALA based fluorescence guided resection (FGR) and PDT can prove to be effective treatment options for this kind of cancer. However insufficient PpIX accumulation hinders the application of FGR and PDT onto the target areas of glioma. 5-ALA-induced PpIX has been associated with fluorescence guidance integrated in the biopsy needle as a novel intraoperative marker. This improved glioma surgery but was not conclusive because the presence of extravasated blood cannot be easily circumvented. The excitation light for PpIX with the usual wavelength of 405 nm is readily absorbed by blood
[50][81]. The poor prognosis for patients with malignant glioma prompted later clinical trials to evaluate whether PDT could offer longer survival than previous treatment options. The PS used was mostly Photofrin
[51][82]. 5-ALA-PDT may offer a significant survival advantage
[52][83], largely attributable to the immunogenic nature of PDT-induced cell death. It may also indicate the efficient destruction of stem-like tumor cells by PDT, but that there is also a considerable risk of treatment-related side effects, probably due to the limited tumor selectivity of PS accumulation
[53][84]. Clinical trials are currently in preparation.
The therapeutic affectivity and safety of 5-ALA-PDT therapy has been investigated in clinical trials. PDT can be applied to the resection cavity after surgery or PDT treatment can be done by administering cylindrical diffuser fibers (CDF). These CDFs are types of optical fibers that are safely administered on the target to ensure the effective illumination of the tumor cells. The research of inserting CDFs into the target area is underway while some clinical researchers have practiced this technique so far
[54][85].
2.2.7. Gynecological Neoplasia
Vulval intraepithelial neoplasia (VIN), which is characterized by a dysplasia of the vulva’s squamous epithelium, is a precursor to vulval squamous cell carcinoma. VIN used to be categorized from 1–3 depending on the severity of the dysplasia. Currently, VIN simply stands for high-grade, which encompasses previous grades 2 and 3. One estimate states that between the years 1973 and 2000, the prevalence of VIN increased by 411%
[55][86].
The standard therapeutic approaches of CO2 laser ablation and surgical excision, with multifocal illness, are both associated with high rates of cancer recurrence. In addition to scarring, vulval anatomical deformity, and constriction of the vaginal introitus, which can result in psychosexual sickness; surgery can have substantial long-term unfavorable repercussions. Therefore, repeated surgical excision can result in chronic and recurring disease in patients with severe disease. The first non-surgical procedure combined 5-ALA with 630 nm non-laser light. Two out of the first ten women showed a histological reaction after receiving a single treatment with a power density of 50 J cm−2. Three out of eight women showed a complete histological response after the single treatment dose was increased to 100 J cm−2 to increase the response rate. Pre-treatment analgesia was used to help patients facing some problems in treatment tolerance. Eighteen women got PDT treatment and sixteen of them reported symptom improvement (89%).
The potential for damaging the surrounding healthy tissue when administering PDT treatment to the vulva was one of the practical difficulties. Photo sensitizers have been used in studies for VIN in a variety of forms, including cream, gel, solution, intravenous route, and so on, for variable times, with an average of three hours for absorption. On an irregular anatomical surface, such as the vulva, with multifocal lesions or a unifocal lesion in a tricky area within skin folds, it is unlikely to maintain a steady absorption of the PS without leakage into the surrounding normal tissue, increasing the risk of damage to the healthy surrounding skin after the application of light. Low doses of PS are believed to have little effect when light is not present, making them non-toxic to the nearby healthy tissue.
2.2.8. Bladder Cancer
Bladder cancer is considered as the fourth most common cancer in men and the eighth most common cancer in women. Most of the bladder cancer cases are superficial (non-muscle invasive). PDT was first reported in 1975, for the treatment of superficial transitional cell carcinoma of the bladder
[56][87]. 5-ALA-PDT is considered the best treatment for bladder cancer because PS can be administered directly intravesically, which directly targets tumors cells and is not able to spread systemically.
The laser system was employed as the light source of a wavelength of 630 nm for photodynamic therapy. Using a laser delivery fiber optic tool, the entire mucosa of the urinary bladder was exposed to diffuse radiation.
According to the literature, the outcomes for patients with bladder tumors were at least comparable to those for actual adjuvant therapy. Intraoperative PDT after TUR reduced the recurrence rate for superficial bladder cancer to 22% at the 1-year follow-up versus 40–80% (according to literature data) for TUR as monotherapy. The results of the study gave promising results for 5-ALA-PDT, suggesting this treatment for people with bladder cancer that is not muscle-invasive, but larger, longer-term trials are required to confirm these results.
2.2.9. Prostate Cancer
A study carried out in a mouse model investigated the treatment effect of a novel form of radio dynamic therapy (RDT) consisting of radiation combined with 5-ALA and carbamide peroxide
[57][89]. The study showed the promising effects of PDT on both primary and post-radiotherapy prostate cancer and consisted of various combinations of high-energy (15-MV) irradiation radiotherapy (RT), 5-ALA, and carbamide peroxide.
According to the authors, this is the first study to investigate the consequences of this specific set of therapies utilized in RDT, as well as the first to use a small animal model to research radio dynamic treatment using 15 MV radiation.
Human prostate cancer cell line PC-3 was bilaterally injected into the mice’s flanks under the skin. The tumors were permitted 1–2 weeks to at least reach the point at which they could be seen on an MR scan (average volume = 180 mm3). The mice were housed at room temperature with a 12 h day/night schedule, unrestricted access to food and water, and were put to death within two weeks of the end of the treatment.
In addition to producing an effect greater than the sum of its parts, RDT was demonstrated to slow tumor growth when compared to RT alone. Furthermore, for each of the tumor size groups into which the data were sorted, RDT was demonstrated to have an impact on tumor growth delay. The findings, therefore, support further research into how radiation energy affects treatment outcomes and show the potential for using clinically relevant, high-radiation energies in radio dynamic therapy. Further studies are required to identify the mechanism by which the peroxide acts in concert with 5-ALA for the treatment and the optimal dose/fractionation scheme for future clinical applications. The effect produced by the RDT designed in this study also highlights the benefit of combining an oxidizing agent to the photo sensitizer and radiation in this treatment.
2.2.10. Actinic Keratoses/Skin Cancer
Squamous cell carcinoma (SCC) in situ and possibly invasive SCC is thought to be caused by actinic keratoses (AK), especially in people with pale skin tones. Many histological characteristics, genetic tumor markers, and p53 alterations are shared by invasive SCCs and AKs. With these details, controlling AK is crucial to get top-notch dermatological care.
Studies on ultraviolet (UV) carcinogenesis in animal models reveal that systemic or large-area topical 5-ALA-PDT treatment can block the growth of SCCs
[58][92]. Similarly, the results of two recently released small clinical trials suggest that large-area (full face) application of 5-ALA-PDT for as little as 1 h in patients with AKs, followed by photo activation with blue light in a manner identical to that used in this study, results in the resolution of AKs with an efficacy like that demonstrated in this and in the larger registration trials of this therapy. The authors also showed a significant improvement in swallowing, pigmentation, skin quality, and Griffiths score, among other photo-damage-related metrics, in perilesional skin. The outcomes of these studies, along with the observation that ALA-PDT can significantly slow UV carcinogenesis (in animal models), imply that non-metastasizing squamous cell carcinoma (NMSC) incidence is unaltered by 5-ALA-PDT, and that the therapy should be investigated for its potential to slow the rate of development of new AKs and perhaps NMSCs. It is obvious that more research is required to address these issues.