Equine piroplasmosis (EP), caused by the hemoparasites Theileria equi, Theileria haneyi, and Babesia caballi, is an important tick-borne disease of equines that is prevalent in most parts of the world. Infection may affect animal welfare and has economic impacts related to limitations in horse transport between endemic and non-endemic regions, reduced performance of sport horses and treatment costs.
- equine piroplasmosis
- Theileria equi
- Babesia caballi
- equine
- genotyping
1. Introduction
Equine piroplasmosis (EP) is a tick-borne disease of equines caused by the eukaryotic hemoparasites Theileria equi, Theileria haneyi, and Babesia caballi that has a considerable veterinary and economic impacts on the horse industry worldwide [1][2][3][4][5]. The parasites belong to the phylum Apicomplexa and to the order Piroplasmida[6]. EP is considered a reportable disease by the World Organization for Animal Health (OIE) (https://www.oie.int/animal-health-in-the-world/oie-listed-diseases-2020/, 15 April 2020). It is estimated that 90% of the global horse population resides in EP-endemic areas, and therefore many studies have investigated the occurrence, prevalence, risk factors, and characteristics of these parasites in different parts of the world.
2. Life Cycle, Vectors, and Transmission
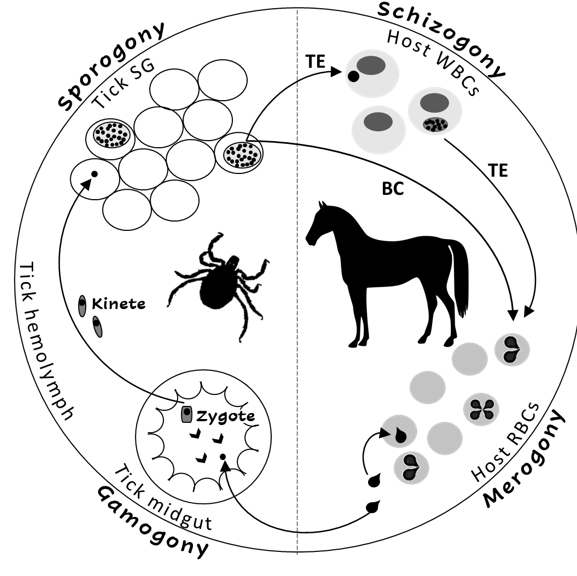
The Theileria and Babesia genera belong to the families Theileriidae and Babesiidae within the phylum Apicomplexa. The life cycles of both parasites include sexual (gamogony) and asexual (sporogony) replicative stages within the tick vector and asexual replicative stages within the equine host[2][3] [2,3]. Asexual replication (merogony) in equine erythrocytes is common to both parasites, and T. equi (and likely, T. haneyi) also undergoes asexual schizogony within equine lymphocytes and monocytes prior to invasion to erythrocytes[7] [7] (Figure 1). The term piroplasmosis derives from the pear-shaped appearance of the intra-erythrocytic stages of these parasites (merozoites). Replication in erythrocytes ultimately leads to cell rupture and the release of merozoites that invade additional cells[2][3][7].
Figure 1. The life cycle of Theileria equi (TE) and Babesia caballi (BC) in the tick vector and in the equine host. RBC—equine red blood cells, WBC—equine while blood cells, SG—tick salivary glands.
The main route of transmission to equids is by tick feeding. Over 30 species of ticks have been described as vectors of one or both T. equi and B. caballi, including the genera Hyalomma, Rhipicephalus Dermacentor, Amblyomma, and Haemaphysalis[8]. Transstadial transmission was recorded for both parasites in several tick species; however, transovarian transmission was only recorded for B. caballi[7]. Therefore, the main reservoir for T. equi is in the equine host, whilst for B. caballi it is the vector ticks[7].
Transplacental transmission in the equine host has been reported for T. equi and may lead to abortion, the birth of a sick foal with peracute neonatal EP, or the birth of unapparent carrier foal[9][10][11][12][13][14][15][16][17][18][19]. In some endemic areas, T. equi is considered to be a major cause of abortion[20][21]; however, the role of this parasite as a cause of abortion is not well established[22] Iatrogenic transmission is also possible; there are several reports of infections resulting from blood transfusions, and from sharing of surgical equipment or needles[2][3][5][20]. However, these types of transmission probably do not have a major role in the epidemiology of EP.
3. Clinical Disease
Clinical disease in EP is mainly attributed to intravascular hemolytic anemia caused by parasite replication and damage to erythrocytes[2][3][20][23]. The clinical signs are similar following infection with both parasite species; however, clinical presentation tends to be more severe in cases of T. equi infection[2][3][20]. The incubation period ranges between 12 and 19 days for T. equi and between 10 and 30 days for B. caballi [2]. Common clinical signs are non-specific and derive from the hemolytic anemia. These include fever, inappetence, icterus, hemoglobinuria, pale mucus membranes (MM), tachycardia, and tachypnea. Thrombocytopenia has also been described. In severe cases, edema and hemorrhage might develop and may eventually lead to organ failure. Gross pathologic findings may include hepatomegaly, splenomegaly, enlarged kidneys, multifocal edema, and hemorrhages[2][3][20][23]. Anecdotal cases of EP-associated hyphema[24], cardiac arrythmias[25], and inflammatory myopathy[26] have also been reported.
Clinical manifestations following infection with either parasite species range from unapparent infection to life threatening disease. Most infected horses remain asymptomatic[2][3][20], while clinically infected horses may develop peracute, acute, subacute, or chronic disease presentations. Peracute disease is life-threatening and has been mostly described in cases of neonatal EP[2][3][20]. Acute disease is characterized by overt presentation of characteristic EP clinical signs, subacute disease manifests milder clinical signs, and chronic disease presents with non-specific signs and mild clinical pathology abnormalities[2][3][20][23][27]. The factors associated with the severity of clinical disease are unknown. Acute disease is more often observed in infections of naïve adult horses, and is less common in equine populations in endemic areas[2][3][20][23]. Stress has been suggested to induce more severe clinical signs, although the evidence to support this assumption is limited[28]. In contrast to T. equi, the newly identified T. haneyi rarely causes clinical signs, even in splenectomized horses[29].
Regardless of the initial clinical presentation, without treatment, horses infected with EP usually remain persistent subclinical (unapparent) carriers for prolonged periods of time. Carriage of T. equi is usually life-long, while B. caballi infection may be self-limiting after up to four years[2][3][20].
4. Immunity, Treatment, and Control
Carriage of parasites usually results in an immune response sufficient to prevent severe disease[2][3]. The precise immune mechanisms involved are not fully elucidated. Both innate and adaptive immunity appear to be necessary for parasite control, and splenectomy leads to severe clinical disease in T. equi-infected horses. Antibodies are first detected seven to 11 days after infection, and peak 30 to 54 days after infection[2][3][30][31][32].
The most widely used treatment for EP is imidocarb dipropionate[2][3][5]. Theileria equi is considered to be more resistant to treatment than B. caballi, and requires higher dosages and longer durations of therapy[33][34][35][36]. Two intramuscular (IM) injections 24 h apart of 2 mg/kg are recommended for the treatment of B. caballi, and four injections 72 h apart of 4 mg/kg are recommended for T. equi [33]. Although this drug is relatively safe, the latter dosage is near its 50% lethal dose (LD50), and may cause adverse signs of toxicity or even death (donkeys being more sensitive than horses) [2][3][35][36]. Since the administration of 4 mg/kg of imidicarb dipropionate often causes colic in horses, animals are often co-treated with flunixin meglumine or buscopan. Although complete parasite clearance is usually possible, several imidocarb diproprionate treatment cycles may be required[36]. Furthermore, imidocarb diproprionate-resistant parasites have been reported[34].
Various other chemotherapeutic agents have been reported to be potentially used against EP, with variable efficacy, mostly in vitro. Among these are anti-malaria compounds[37][38][39], antimicrobial agents[40][41][42][43][44][45][41][42][43][44], parasite metabolism inhibitors[34][46][47][48][49][50][51][52][53], replication inhibitors[54][55], pyrimidine synthesis inhibitors[56][57], and various plant-derived compounds[58][59][60][61][62][63]. However, most of these options have never been tested in vivo, and none is widely used.
Since no effective, commercially available vaccines against EP are yet available, control is based on a combination of drug therapy, vector control, and restricted transport of infected horses. The aims of treatment and control strategies differ between endemic and non-endemic regions. In non-endemic areas the aim is to keep the area disease-free. Thus, treatment of infected horses is aimed at complete clearance of infection, while control is mainly based on monitoring and restricting the entrance of infected horses. Several non-endemic countries, including the United States, Australia, and Japan deny entrance of seropositive horses, and either export, quarantine, or euthanize any positive animal within the country[1][2][3][5][20][64][65]. In addition to quarantine, horses transported from endemic to non-endemic areas usually require treatment with acaricides to prevent introduction of vector ticks (https://www.oie.int/index.php?id=169&L=0&htmfile=chapitre_equine_piroplasmosis.htm). In endemic areas, unapparent carriage and the development of premonition are usually encouraged, rather than parasite clearance, to prevent clinical outbreaks. Thus, treatment is usually aimed only to reduce clinical sings of acute infection, while strategic use of acaricides is recommended to reduce, but not eliminate, exposure to ticks[1][2][3][5][20].
5. Diagnosis
Diagnosis of EP infection is important to identify unapparent carriers, especially prior to transport into non-endemic areas, and to identify EP as a cause of disease clinically ill animals, especially due to the non-specific nature of clinical signs in EP infection. Various diagnostic techniques have been reported based on clinical signs, microscopic examination, culture, serology, and molecular assays[2][3][5][66].
Traditionally, identification of piriform parasites in Giemsa-stained blood smears was the diagnostic method of choice in clinical cases[2][3][5][67]. However, the sensitivity of this method is low, leading to false negative results in many chronic and subclinical cases, when parasite loads are low. In vitro culture methods proved more sensitive and specific; however, these methods are time-consuming,require fresh blood samples and skilled personnel, and therefore are not frequently used as routine diagnostic tests[2][3][5][20][66][68][69][67][70][71].
Serological diagnosis has better sensitivity and specificity for the detection of unapparent carrier horses; however, these assays do not provide information on current parasite load for interpretation of clinical disease states. Several EP-specific serologic assays, comprised of various methods, including a complement fixation test (CFT) [72][73], an indirect immunoflorescent antibody test (IFAT)[72][73], and an enzyme-linked immunosorbent assay (ELISA)[74][75][76][77] have been developed. The CFT is very specific; however, it may give false negative results, especially after treatment and with chronicity, since IgG(T) is not complement-fixing. IFAT is more sensitive than the CFT and remains positive in chronic cases; however, interpretation of the results is subjective and difficult to standardize[2][3][5][20][65][72][73]. Different ELISA tests were developed to detect EP infection, including an indirect ELISA (iELISA)[74] and a competitive ELISA (cELISA)[78]. To improve the standardization and performance of these tests, several cELISA assays were developed using purified recombinant antigens, and are currently the United States Department of Agriculture (USDA) and OIE recommended tests for international horse transport screening. The use of a single epitope also reduces the chance of cross-reactivity between the parasites. The immunodominant T. equi surface antigens equine merozoite antigen (ema)-1 and ema-2, and the B. caballi rhoptry-associated protein (rap)-1 were successfully used and proven superior to IFAT and CFT in several studies [2][3][5][20][74][65][76][77][79][80][81]. Nevertheless, some heterogeneity has been recorded between isolates, and the USDA-approved B. caballi rap-1 cELISA assay did not detect infected horses in South-Africa and in the Middle East [82][83][84].
Molecular diagnosis, based on the detection of parasite DNA in equine blood by polymerase chain reaction (PCR), is gaining popularity for the detection of parasites in both clinical and carrier animals. These methods are more sensitive than microscopic examination, and more clinically useful than serology, since they represent current infection. These methods can also be designed to distinguish between parasite species or genotypes. Currently, these methods are more often used for research than in clinical practice[2][3][5][20][65][65][85][86][87][85][88][89][90][91][92]. Numerous assays targeting one or multiple EP parasites, including conventional PCR[87][85] , nested PCR (nPCR) [90][93], real-time PCR (rtPCR)[82][94][95][96][97][98][99], multiplex PCR (mPCR)[87][89], reverse line blot (RLB)[100][101], and loop mediated isothermal amplification (LAMP)[85][88][91], have been developed. Several of these assays were determined to have high sensitivity, with a detection limit of 10−7% parasitized erythrocytes (PE) [2][3][5][20][68][85][86][87][85][88][89][90][91][92]. Quantitative methods, such as rtPCR (qPCR), have also been developed, but are mostly applied to increase the sensitivity of parasite detection, and are rarely used to evaluate parasite loads[94][95][96][97][98][99][102].
6. Epidemiology
The transmission dynamics of T. equi and B. caballi are different. In endemic areas, animals are usually exposed at a young age to both parasites and develop premonition. Carriage of T. equi is usually life-long; thus, the observed prevalence increases with age and the host is the main reservoir of parasites. The prevalence of B. caballi, on the other hand, does not increase with age and is higher in younger animals. Clearance of B. caballi is possible, and the parasite is transovarially transmitted by ticks, suggesting the main reservoir of this parasite is the tick [2][3][5][8][103][104][105][106][2,3,5,8,20,113,123–126].
EP is endemic in most parts of the world where competent tick vectors are present. Few countries are considered non-endemic, including the US and Canada, the United Kingdom (UK) and Ireland, Northern Europe, Iceland and Greenland, Singapore, Japan, New Zealand, and Australia. In some of these countries, EP has been reported, but is limited to specific areas and is not widespread or endemic[2][3][5][8][103].
The only risk factors consistently associated with EP infection are management practices and tick exposure. Other factors, including host species, breed, age, sex, and activity, have been inconsistently associated with infection (reviewed in:[5][103]. Although most EP-endemic areas are within tropical and temperate regions, recent global warming and increased global transportation have led to the spread of both parasites and vectors to previously non-endemic areas, such as the UK[107]. Some of these areas are suitable habitats for potential vector ticks and are therefore susceptible to epizootic disease spread—hence the significance of OIE monitoring of the distribution and spread of EP, a summary of which is available through the OIE’s new World Animal Health Information Database (WAHIS) (https://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home, 15 April 2020).
Clinical manifestation of EP is less common in endemic areas, since early exposure is likely to induce protection [2][3][20][23]. However, clinical cases have been reported in resident horses in both non-endemic (Poland, The Netherlands, USA)[108][109][110][111] and in endemic areas (Israel, Italy, Romania, Spain)[25][112][113][114][115]. This highlights the fact that in areas which are considered endemic, there are sub-populations of horses that differ in their exposure to vector ticks, and subsequently to infection with EP and to the development of premonition. These sub-populations should be approached differently in application of preventive measures and treatment to reduce the chance of clinical disease[1][3][5][10][105].
Both parasites are endemic in similar areas, although T. equi is more frequently reported and is usually more prevalent than B. caballi. This may reflect the different transmission cycles of these parasites, since the main reservoirs of B. caballi and T. equi are in ticks and horses, respectively[2][3][8][20][116]. In addition, parasite loads in both clinically affected horses and in unapparent carriers are usually higher in cases of T. equi infection than in B. caballi infection, increasing the odds it will be detected by a diagnostic test [2][3][114].
In addition to horses, both parasites have been reported in other equids including domestic donkeys [117][118][119][120][121][122][123][124][125][126][127][128], wild donkeys[123][129], mules[117][119][120][121][125], and zebras [102][123][129][130][131][132][133][134]; and in non-equids, including dogs[135][131][132][133][134][136][137][138][139][140] [135][136][137][138][139][140], camels[141][142], cattle[143], and a tapir [144](recently reviewed in: [5][123]). Donkeys are considered more resistant to infection than horses[126]; however, this assumption is not well established, since the data regarding domestic equids (donkeys and mules) is less comprehensive than in horses, and many surveys use a population of different equine species. Reports in other animals are anecdotal, and the role of other species as a reservoir of EP has not been demonstrated. All reports describing EP in other animals originate from endemic areas of EP in horses.
7. Genotyping
Since their discovery in 1901[145], the taxonomy of these parasites has been challenged, and they have been re-named and re-classified several times. Currently, B. caballi is considred a “true Babesia,” while T. equi (formerly: B. equi) has been classified as Theileria based on its extra-erythrocytic life stage in lymphocytes and the absence of transovarian transmission in ticks[116]. Molecular investigations indicate that it possesses characteristics of both Babesia and Theileria, and is possibly placed between the two[6][146][147][148][149]. The full genome of T. equi was constructed from an American strain, which had been used in numerous phylogenetic studies evaluating the genetic diversity between and within piroplasm species[148]. Considerable genetic variation has been found within T. equi, and recent discoveries of novel, closely related, species, including T. haneyi, suggest that current classification of T. equi may include several distinct organisms[4][150].
Phylogenetic studies investigating inter-species diversity between piroplasms have mainly focused on the 18S rRNA[6][150][151][146][149][152], β-tubulin[153], mitochondrial genes[147], and ema-1 genes[154][155][242,243], while intra-species diversity and genotyping mainly focused on the 18S rRNA [17][83][100][102][112][156][150][157][114][158][159], T. equi ema-1 [95][107][121][160][114][159], and B. caballi rap-1[82][83][84][112][161][114] genes. Five T. equi 18S rRNA genotypes (A–E) have been identified up to date[89][100][102][162][163][164], and three (A–C) ema-1 genotypes[95]. Three B. caballi 18S rRNA genotypes have been described (A, B1, and B2)[94][100] [114], along with three rap-1 genotypes (A1, A2, B)[82][83][84]. Recently, the identification of T. equi by its 18S rRNA gene has been scrutinized following the discovery of several new species that were indistinguishable from T. equi based on this locus, suggesting this classification may actually represent several distinct species[4][150]. No specific genotype had been found to be linked to increased virulence; however, two unrelated studies reported a higher frequency of clinically affected horses are infected with T. equi 18S rRNA genotype A[107] [114].
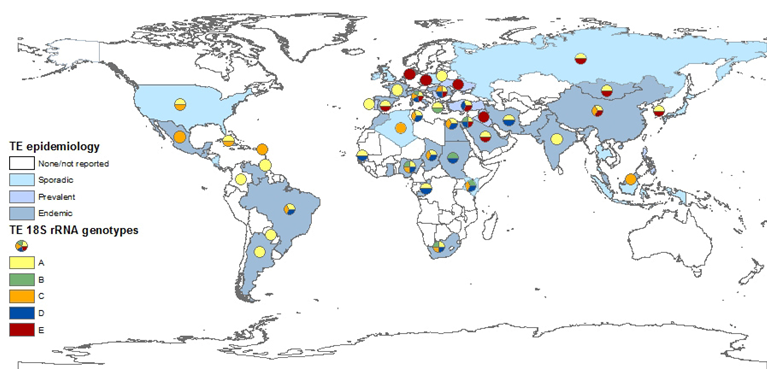
Despite concerns raised that T. equi 18S rRNA gene may not allow clear distinction between closely related Theileria species[4][150], the analysis of its various sequences in the GenBank database provided the best basis to determine T. equi genotype distribution (Figure 2), while the ema-1 and ema-2 loci are more conserved. The most widely distributed T. equi 18S rRNA genotype is genotype A. This genotype has been isolated in most countries and on all continents. This genotype is also the only one that has been fully sequenced. Although there is no concrete evidence linking any specific genotype to parasite virulence, at least two studies suggest infection with genotype A leads to more severe clinical disease[107][114] , and this correlation has also been described during several outbreaks[158]. Moreover, this is the main genotype isolated from ticks[165]] and the only genotype isolated from dogs. Genotype C is also widely distributed, and was also found on all continents. In the Americas, genotypes A and C are the predominant genotypes. Genotype D was mainly found in Africa, the Mediterranean region, and the Middle East, and had not been isolated from Northern Europe, the Far East, North and Central America, or the Caribbean region. Genotype E, on the other hand, is mainly found in the Far East, Northern and Eastern Europe and the Middle East, but not in Africa, America or the Caribbean. Genotype B was only detected in Africa and the Mediterranean region. The differences in the distribution of each genotype is important in understanding the spread of parasites and the infection dynamics within and between equine populations. Recent studies showed that in endemic areas, many horses are co-infected with several genotypes of T. equi, and that the predominant genotype or genotypes differ between equine hosts and subpopulations[102][105][130][166]. Co-infection is also possible with other related species, including T. haneyi[28] and B. caballi (Table 1). The significance of this co-infection and the relations between parasites or genotypes within the host should be further investigated, since it is likely to be a part of maintaining the enzootic stability in endemic areas.
Recent work demonstrated a correlation between B. caballi 18S rRNA and rap-1 genotypes [114], making the classification more robust. However, since relatively fewer studies provided genotypic characterization of B. caballi, additional molecular data from various locations should be gathered and analyzed in order to understand the global molecular epidemiology of this parasite.
Figure 2. Global prevalence of T. equi, and the distribution of T. equi 18S rRNA genotypes. The map was constructed based on epidemiological data published in the last 20 years (2000–2019). Endemic: over 30%, prevalent: 10–29%, sporadic: under 10% or singular outbreaks. Genotyping was performed on all sequences submitted to GenBank and classification was based on previously reported clades.
Conclusion
EP is endemic in most parts of the world, and is spreading further into more temperate climate zones previously considered parasite-free. Basing the diagnosis on advanced molecular tools and increasing the understanding of the differences between genotypes will enable to better control these important pathogens, thus, reducing their clinical and economic impact.
References
- Friedhoff, K.T.; Tenter, A.M.; Muller, I. Haemoparasites of equines: Impact on international trade of horses. Rev. Sci. Tech. 1990, 9, 1187–1194.
- Rothschild, C.M. Equine piroplasmosis. J. Equine Vet. Sci. 2013, 23, 115–120.
- Wise, L.N.; Kappmeyer, L.S.; Mealey, R.H.; Knowles, D.P. Review of equine piroplasmosis. J. Vet. Intern. Med. 2013, 27, 1334–1346, doi:10.1111/jvim.12168.
- Knowles, D.P.; Kappmeyer, L.S.; Haney, D.; Herndon, D.R.; Fry, L.M.; Munro, J.B.; Sears, K.; Ueti, M.W.; Wise, L.N.; Silva, M.; et al. Discovery of a novel species, Theileria haneyi n. sp., infective to equids, highlights exceptional genomic diversity within the genus Theileria: Implications for apicomplexan parasite surveillance. Int. J. Parasitol. 2018, 48, 679–690, doi:10.1016/j.ijpara.2018.03.010.
- Onyiche, T.E.; Suganuma, K.; Igarashi, I.; Yokoyama, N.; Xuan, X.; Thekisoe, O. A Review on Equine Piroplasmosis: Epidemiology, Vector Ecology, Risk Factors, Host Immunity, Diagnosis and Control. Int. J. Environ. Res. Public Health 2019, 16, doi:10.3390/ijerph16101736.
- Allsopp, M.; Cavalier-Smith, T.; de Waal, D.; Allsopp, B. Phylogeny and evolution of the piroplasms. Parasitology 1994, 108, 147–152.
- Scoles, G.A.; Ueti, M.W. Vector ecology of equine piroplasmosis. Annu. Rev. Entomol. 2015, 60, 561–580, doi:10.1146/annurev-ento-010814-021110.
- Donnelly, J.; Phipps, L.P.; Watkins, K.L. Evidence of Maternal Antibodies to Babesia-Equi and Babesia-Caballi in Foals of Seropositive Mares. Equine Vet. J. 1982, 14, 126–128, doi:10.1111/j.2042-3306.1982.tb02365.x.
- Georges, K.C.; Ezeokoli, C.D.; Sparagano, O.; Pargass, I.; Campbell, M.; D’Abadie, R.; Yabsley, M.J. A case of transplacental transmission of Theileria equi in a foal in Trinidad. Vet. Parasitol. 2011, 175, 363–366, doi:10.1016/j.vetpar.2010.10.019.
- Kumar, S.; Kumar, R.; Gupta, A.K.; Dwivedi, S.K. Passive transfer of Theileria equi antibodies to neonate foals of immune tolerant mares. Vet. Parasitol. 2008, 151, 80–85, doi:10.1016/j.vetpar.2007.10.001.
- Levi, M.M.; Tirosh-Levy, S.; Dahan, R.; Berlin, D.; Steinman, A.; Edery, N.; Savitski, I.; Lebovich, B.; Knowles, D.; Suarez, C.E.; et al. First Detection of Diffuse and Cerebral Theileria equi Infection in Neonatal Filly. J. Equine Vet. Sci. 2018, 60, 23–28, doi:10.1016/j.jevs.2017.10.016.
- Oliveira, A.; Pinheiro, G.; Souza, T.; Flecher, M.; Santos, R. Abortion in association with transplacental Theileria equi infection in a mare from the State of Espírito Santo, southeast Brazil: Case report. Arq. Bras. Med. Vet. Zootec. 2019, 71, 369–373.
- Phipps, L.P.; Otter, A. Transplacental transmission of Theileria equi in two foals born and reared in the United Kingdom. Vet. Rec 2004, 154, 406–408, doi:10.1136/vr.154.13.406.
- Sant, C.; Allicock, O.M.; d’Abadie, R.; Charles, R.A.; Georges, K. Phylogenetic analysis of Theileria equi and Babesia caballi sequences from thoroughbred mares and foals in Trinidad. Parasitol. Res. 2019, 118, 1171–1177, doi:10.1007/s00436-019-06240-x.
- Sant, C.; d’Abadie, R.; Pargass, I.; Basu, A.K.; Asgarali, Z.; Charles, R.A.; Georges, K.C. Prospective study investigating transplacental transmission of equine piroplasmosis in thoroughbred foals in Trinidad. Vet. Parasitol. 2016, 226, 132–137, doi:10.1016/j.vetpar.2016.07.008.
- Sudan, V.; Jaiswal, A.K.; Srivastava, A.; Saxena, A.; Shanker, D. A rare clinical presentation of transplacental transmission and subsequent abortion by Babesia (Theileria) equi in a mare. J. Parasit. Dis. 2015, 39, 336–338, doi:10.1007/s12639-013-0337-y.
- De Waal, D. Equine piroplasmosis: A review. Brit. Vet. J. 1992, 148, 6–14.
- Sant, C.; d’Abadie, R.; Pargass, I.; Basu, A.K.; Asgarali, Z.; Charles, R.A.; Georges, K.C. Prospective study investigating transplacental transmission of equine piroplasmosis in thoroughbred foals in Trinidad. Vet. Parasitol. 2016, 226, 132–137, doi:10.1016/j.vetpar.2016.07.008.
- Sudan, V.; Jaiswal, A.K.; Srivastava, A.; Saxena, A.; Shanker, D. A rare clinical presentation of transplacental transmission and subsequent abortion by Babesia (Theileria) equi in a mare. J. Parasit. Dis. 2015, 39, 336–338, doi:10.1007/s12639-013-0337-y.
- Lewis, B.D.; Penzhorn, B.L.; Volkmann, D.H. Could treatment of pregnant mares prevent abortions due to equine piroplasmosis? J. S. Afr. Vet. Assoc. 1999, 70, 90–91.
- Tirosh-Levy, S.; Gottlieb, Y.; Mimoun, L.; Mazuz, M.L.; Steinman, A. Transplacental Transmission of Theileria equi is not a Common Cause of Abortions and Infection of Foals in Israel. Animals 2020, 10, doi:10.3390/ani10020341.
- Tirosh-Levy, S.; Gottlieb, Y.; Mimoun, L.; Mazuz, M.L.; Steinman, A. Transplacental Transmission of Theileria equi is not a Common Cause of Abortions and Infection of Foals in Israel. Animals 2020, 10, doi:10.3390/ani10020341.
- Zobba, R.; Ardu, M.; Niccolini, S.; Chessa, B.; Manna, L.; Cocco, R.; Parpaglia, M.L.P. Clinical and laboratory findings in equine piroplasmosis. J. Equine Vet. Sci. 2008, 28, 301–308.
- Prasad, A.; Kumar, V.; Kumar, B. First Report of Acute Bilateral Hyphema in a Theileria equi-Infected Kathiawari Horse. J. Equine Vet. Sci. 2019, 77, 72–74, doi:10.1016/j.jevs.2019.02.021.
- Diana, A.; Guglielmini, C.; Candini, D.; Pietra, M.; Cipone, M. Cardiac arrhythmias associated with piroplasmosis in the horse: A case report. Vet. J. 2007, 174, 193–195, doi:10.1016/j.tvjl.2006.04.003.
- Pasolini, M.P.; Pagano, T.B.; Costagliola, A.; Biase, D.; Lamagna, B.; Auletta, L.; Fatone, G.; Greco, M.; Coluccia, P.; Vincenzo, V.; et al. Inflammatory Myopathy in Horses with Chronic Piroplasmosis. Vet. Pathol. 2018, 55, 133–143, doi:10.1177/0300985817716262.
- Padalino, B.; Rosanowski, S.M.; di Bella, C.; Lacinio, R.; Rubino, G.T.R. Piroplasmosis in Italian Standardbred Horses: 15 Years of Surveillance Data. J. Equine Vet. Sci 2019, 83, 102813, doi:10.1016/j.jevs.2019.102813.
- Tirosh-Levy, S.; Gottlieb, Y.; Steinman, A. Stress conditions do not affect Theileria equi parasitemia levels in sub-clinically infected horses. Tick Tick-Borne Dis. 2020, doi:10.1016/j.ttbdis.2020.101384.
- Sears, K.P.; Kappmeyer, L.S.; Wise, L.N.; Silva, M.; Ueti, M.W.; White, S.; Reif, K.E.; Knowles, D.P. Infection dynamics of Theileria equi and Theileria haneyi, a newly discovered apicomplexan of the horse. Vet. Parasitol. 2019, 271, 68–75, doi:10.1016/j.vetpar.2019.06.009.
- Knowles, D.P., Jr.; Kappmeyer, L.S.; Stiller, D.; Hennager, S.G.; Perryman, L.E. Antibody to a recombinant merozoite protein epitope identifies horses infected with Babesia equi. J. Clin. Microbiol. 1992, 30, 3122–3126.
- Lewis, M.J.; Wagner, B.; Woof, J.M. The different effector function capabilities of the seven equine IgG subclasses have implications for vaccine strategies. Mol. Immunol. 2008, 45, 818–827.
- Mealey, R.H.; Kappmeyer, L.S.; Ueti, M.W.; Wagner, B.; Knowles, D.P. Protective effects of passively transferred merozoite-specific antibodies against Theileria equi in horses with severe combined immunodeficiency. Clin. Vaccine Immunol. 2012, 19, 100–104, doi:10.1128/CVI.05301-11.
- Butler, C.M.; Nijhof, A.M.; van der Kolk, J.H.; de Haseth, O.B.; Taoufik, A.; Jongejan, F.; Houwers, D.J. Repeated high dose imidocarb dipropionate treatment did not eliminate Babesia caballi from naturally infected horses as determined by PCR-reverse line blot hybridization. Vet. Parasitol. 2008, 151, 320–322, doi:10.1016/j.vetpar.2007.11.010.
- Hines, S.A.; Ramsay, J.D.; Kappmeyer, L.S.; Lau, A.O.; Ojo, K.K.; van Voorhis, W.C.; Knowles, D.P.; Mealey, R.H. Theileria equi isolates vary in susceptibility to imidocarb dipropionate but demonstrate uniform in vitro susceptibility to a bumped kinase inhibitor. Parasites Vectors 2015, 8, 33, doi:10.1186/s13071-014-0611-6.
- Grause, J.F.; Ueti, M.W.; Nelson, J.T.; Knowles, D.P.; Kappmeyer, L.S.; Bunn, T.O. Efficacy of imidocarb dipropionate in eliminating Theileria equi from experimentally infected horses. Vet. J. 2013, 196, 541–546.
- Schwint, O.N.; Ueti, M.W.; Palmer, G.H.; Kappmeyer, L.S.; Hines, M.T.; Cordes, R.T.; Knowles, D.P.; Scoles, G.A. Imidocarb dipropionate clears persistent Babesia caballi infection with elimination of transmission potential. Antimicrob. Agents Chemother. 2009, 53, 4327–4332, doi:10.1128/AAC.00404-09.
- Nugraha, A.B.; Tuvshintulga, B.; Guswanto, A.; Tayebwa, D.S.; Rizk, M.A.; Gantuya, S.; El-Saber Batiha, G.; Beshbishy, A.M.; Sivakumar, T.; Yokoyama, N.; et al. Screening the Medicines for Malaria Venture Pathogen Box against piroplasm parasites. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 84–90, doi:10.1016/j.ijpddr.2019.06.004.
- Nagai, A.; Yokoyama, N.; Matsuo, T.; Bork, S.; Hirata, H.; Xuan, X.; Zhu, Y.; Claveria, F.G.; Fujisaki, K.; Igarashi, I. Growth-inhibitory effects of artesunate, pyrimethamine, and pamaquine against Babesia equi and Babesia caballi in in vitro cultures. Antimicrob. Agents Chemother. 2003, 47, 800–803, doi:10.1128/aac.47.2.800-803.2003.
- Rizk, M.A.; El-Sayed, S.A.E.; El-Khodery, S.; Yokoyama, N.; Igarashi, I. Discovering the in vitro potent inhibitors against Babesia and Theileria parasites by repurposing the Malaria Box: A review. Vet. Parasitol. 2019, 274, 108895, doi:10.1016/j.vetpar.2019.07.003.
- Tuvshintulga, B.; AbouLaila, M.; Davaasuren, B.; Ishiyama, A.; Sivakumar, T.; Yokoyama, N.; Iwatsuki, M.; Otoguro, K.; Omura, S.; Igarashi, I. Clofazimine Inhibits the Growth of Babesia and Theileria Parasites In Vitro and In Vivo. Antimicrob. Agents Chemother. 2016, 60, 2739–2746, doi:10.1128/AAC.01614-15.
- Rizk, M.A.; El-Sayed, S.A.; AbouLaila, M.; Yokoyama, N.; Igarashi, I. Evaluation of the inhibitory effect of N-acetyl-L-cysteine on Babesia and Theileria parasites. Exp. Parasitol. 2017, 179, 43–48, doi:10.1016/j.exppara.2017.06.003.
- Omar, M.A.; Salama, A.; Elsify, A.; Rizk, M.A.; Al-Aboody, M.S.; Aboulaila, M.; El-Sayed, S.A.; Igarashi, I. Evaluation of in vitro inhibitory effect of enoxacin on Babesia and Theileria parasites. Exp. Parasitol. 2016, 161, 62–67, doi:10.1016/j.exppara.2015.12.016.
- Salama, A.A.; Aboulaila, M.; Moussa, A.A.; Nayel, M.A.; El-Sify, A.; Terkawi, M.A.; Hassan, H.Y.; Yokoyama, N.; Igarashi, I. Evaluation of in vitro and in vivo inhibitory effects of fusidic acid on Babesia and Theileria parasites. Vet. Parasitol. 2013, 191, 1–10, doi:10.1016/j.vetpar.2012.08.022.
- Ikadai, H.; Tanaka, T.; Shibahara, N.; Tanaka, H.; Matsuu, A.; Kudo, N.; Shimazaki, K.; Igarashi, I.; Oyamada, T. Inhibitory effect of lactoferrin on in vitro growth of Babesia caballi. Am. J. Trop. Med. Hyg. 2005, 73, 710–712.
- Silva, M.G.; Villarino, N.F.; Knowles, D.P.; Suarez, C.E. Assessment of Draxxin((R)) (tulathromycin) as an inhibitor of in vitro growth of Babesia bovis, Babesia bigemina and Theileria equi. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 265–270, doi:10.1016/j.ijpddr.2018.04.004.
- Maji, C.; Goel, P.; Suthar, A.; Mandal, K.D.; Gopalakrishnan, A.; Kumar, R.; Tripathi, B.N.; Kumar, S. Lumefantrine and o-choline—Parasite metabolism specific drug molecules inhibited in vitro growth of Theileria equi and Babesia caballi in MASP culture system. Tick Tick-Borne Dis. 2019, 10, 568–574, doi:10.1016/j.ttbdis.2019.01.004.
- Gimenez, F.; Hines, S.A.; Evanoff, R.; Ojo, K.K.; van Voorhis, W.C.; Maly, D.J.; Vidadala, R.S.R.; Mealey, R.H. In vitro growth inhibition of Theileria equi by bumped kinase inhibitors. Vet. Parasitol. 2018, 251, 90–94, doi:10.1016/j.vetpar.2017.12.024.
- Silva, M.G.; Knowles, D.P.; Antunes, S.; Domingos, A.; Esteves, M.A.; Suarez, C.E. Inhibition of the in vitro growth of Babesia bigemina, Babesia caballi and Theileria equi parasites by trifluralin analogues. Tick Tick-Borne Dis. 2017, 8, 593–597, doi:10.1016/j.ttbdis.2017.04.002.
- Gopalakrishnan, A.; Maji, C.; Dahiya, R.K.; Suthar, A.; Kumar, R.; Gupta, A.K.; Dimri, U.; Kumar, S. In vitro growth inhibitory efficacy of some target specific novel drug molecules against Theileria equi. Vet. Parasitol. 2016, 217, 1–6, doi:10.1016/j.vetpar.2015.12.024.
- AbouLaila, M.; Batadoj, D.; Salama, A.; Munkhjargal, T.; Ichikawa-Seki, M.; Terkawi, M.A.; Yokoyama, N.; Igarashi, I. Evaluation of the inhibitory effects of miltefosine on the growth of Babesia and Theileria parasites. Vet. Parasitol. 2014, 204, 104–110, doi:10.1016/j.vetpar.2014.05.023.
- Aboulaila, M.; Munkhjargal, T.; Sivakumar, T.; Ueno, A.; Nakano, Y.; Yokoyama, M.; Yoshinari, T.; Nagano, D.; Katayama, K.; El-Bahy, N.; et al. Apicoplast-targeting antibacterials inhibit the growth of Babesia parasites. Antimicrob. Agents Chemother. 2012, 56, 3196–3206, doi:10.1128/AAC.05488-11.
- Wise, L.N.; Ueti, M.W.; Kappmeyer, L.S.; Hines, M.T.; White, S.N.; Davis, W.; Knowles, D.P. In vitro activity of ponazuril against Theileria equi. Vet. Parasitol. 2012, 185, 282–285, doi:10.1016/j.vetpar.2011.10.036.
- Aboulaila, M.; Nakamura, K.; Govind, Y.; Yokoyama, N.; Igarashi, I. Evaluation of the in vitro growth-inhibitory effect of epoxomicin on Babesia parasites. Vet. Parasitol. 2010, 167, 19–27, doi:10.1016/j.vetpar.2009.09.049.
- Tayebwa, D.S.; Tuvshintulga, B.; Guswanto, A.; Nugraha, A.B.; Batiha, G.E.; Gantuya, S.; Rizk, M.A.; Vudriko, P.; Sivakumar, T.; Yokoyama, N.; et al. The effects of nitidine chloride and camptothecin on the growth of Babesia and Theileria parasites. Tick Tick-Borne Dis. 2018, 9, 1192–1201, doi:10.1016/j.ttbdis.2018.04.019.
- Guswanto, A.; Nugraha, A.B.; Tuvshintulga, B.; Tayebwa, D.S.; Rizk, M.A.; Batiha, G.E.; Gantuya, S.; Sivakumar, T.; Yokoyama, N.; Igarashi, I. 17-DMAG inhibits the multiplication of several Babesia species and Theileria equi on in vitro cultures, and Babesia microti in mice. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 104–111, doi:10.1016/j.ijpddr.2018.02.005.
- Boschi, D.; Pippione, A.C.; Sainas, S.; Lolli, M.L. Dihydroorotate dehydrogenase inhibitors in anti-infective drug research. Eur. J. Med. Chem. 2019, 183, 111681, doi:10.1016/j.ejmech.2019.111681.
- Kamyingkird, K.; Cao, S.; Tuvshintulga, B.; Salama, A.; Mousa, A.A.; Efstratiou, A.; Nishikawa, Y.; Yokoyama, N.; Igarashi, I.; Xuan, X. Effects of dihydroorotate dehydrogenase (DHODH) inhibitors on the growth of Theileria equi and Babesia caballi in vitro. Exp. Parasitol. 2017, 176, 59–65, doi:10.1016/j.exppara.2017.03.002.
- Beshbishy, A.M.; Batiha, G.E.; Yokoyama, N.; Igarashi, I. Ellagic acid microspheres restrict the growth of Babesia and Theileria in vitro and Babesia microti in vivo. Parasites Vectors 2019, 12, 269, doi:10.1186/s13071-019-3520-x.
- Batiha, G.E.; Beshbishy, A.M.; Tayebwa, D.S.; Shaheen, H.M.; Yokoyama, N.; Igarashi, I. Inhibitory effects of Syzygium aromaticum and Camellia sinensis methanolic extracts on the growth of Babesia and Theileria parasites. Tick Tick-Borne Dis. 2019, 10, 949–958, doi:10.1016/j.ttbdis.2019.04.016.
- Ganchimeg, D.; Batbold, B.; Murata, T.; Davaapurev, B.O.; Munkhjargal, T.; Tuvshintulga, B.; Suganuma, K.; Igarashi, I.; Buyankhishig, B.; Sasaki, K.; et al. Flavonoids isolated from the flowers of Pulsatilla flavescens and their anti-piroplasm activity. J. Nat. Med. 2019, 73, 633–640, doi:10.1007/s11418-019-01294-8.
- Badral, D.; Odonbayar, B.; Murata, T.; Munkhjargal, T.; Tuvshintulga, B.; Igarashi, I.; Suganuma, K.; Inoue, N.; Brantner, A.H.; Odontuya, G.; et al. Flavonoid and Galloyl Glycosides Isolated from Saxifraga spinulosa and Their Antioxidative and Inhibitory Activities against Species That Cause Piroplasmosis. J. Nat. Prod. 2017, 80, 2416–2423, doi:10.1021/acs.jnatprod.7b00142.
- El-Sayed, S.A.E.; Rizk, M.A.; Yokoyama, N.; Igarashi, I. Evaluation of the in vitro and in vivo inhibitory effect of thymoquinone on piroplasm parasites. Parasites Vectors 2019, 12, 37, doi:10.1186/s13071-019-3296-z.
- Naidoo, V.; Zweygarth, E.; Eloff, J.N.; Swan, G.E. Identification of anti-babesial activity for four ethnoveterinary plants in vitro. Vet. Parasitol. 2005, 130, 9–13, doi:10.1016/j.vetpar.2005.03.001.
- Equine piroplasmosis visits Australia in 2000. Aust. Vet. J. 2000, 78, 380, doi:10.1111/j.1751-0813.2000.tb11817.x.
- Martin, R. Equine piroplasmosis: The temporary importation of seropositive horses into Australia. Aust. Vet. J. 1999, 77, 308–309, doi:10.1111/j.1751-0813.1999.tb10269.x.
- Bruning, A. Equine piroplasmosis an update on diagnosis, treatment and prevention. Br. Vet. J. 1996, 152, 139–151, doi:10.1016/s0007-1935(96)80070-4.
- Zweygarth, E.; Josemans, A.I. L-cysteine replaces microaerophilous culture conditions for the in vitro initiation of Theileria equi. Parasitol. Res. 2014, 113, 433–435, doi:10.1007/s00436-013-3672-0.
- Bonfini, B.; Semproni, G.; Savini, G. Use of an in vitro culture system to detect Theileria equi strains from infected equids and/or reservoirs. Vet. Ital. 2006, 42, 217–223, 209-215.
- Mans, B.J.; Pienaar, R.; Latif, A.A. A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. Parasites Wildl. 2015, 4, 104–118, doi:10.1016/j.ijppaw.2014.12.006.
- Zweygarth, E.; Lopez-Rebollar, L.M.; Nurton, J.; Guthrie, A.J. Culture, isolation and propagation of Babesia caballi from naturally infected horses. Parasitol. Res. 2002, 88, 460–462, doi:10.1007/s00436-002-0609-4.
- Friedhoff, K.; Soule, C. An account on equine babesioses. Rev. Sci. Tech. 1996, 15, 1191.
- Ogunremi, O.; Halbert, G.; Mainar-Jaime, R.; Benjamin, J.; Pfister, K.; Lopez-Rebollar, L.; Georgiadis, M.P. Accuracy of an indirect fluorescent-antibody test and of a complement-fixation test for the diagnosis of Babesia caballi in field samples from horses. Prev. Vet. Med. 2008, 83, 41–51, doi:10.1016/j.prevetmed.2007.06.009.
- Ogunremi, O.; Georgiadis, M.P.; Halbert, G.; Benjamin, J.; Pfister, K.; Lopez-Rebollar, L. Validation of the indirect fluorescent antibody and the complement fixation tests for the diagnosis of Theileria equi. Vet. Parasitol. 2007, 148, 102–108, doi:10.1016/j.vetpar.2007.06.006.
- Asenzo, G.; Wilkowsky, S.; Barrandeguy, M.; Mesplet, M.; Benitez, D.; Florin-Christensen, M. Development of an indirect ELISA for the diagnosis of equine piroplasmosis. Ann. N. Y. Acad. Sci. 2008, 1149, 235–238, doi:10.1196/annals.1428.029.
- Baldani, C.D.; Nakaghi, A.C.; Machado, R.Z. Occurrence of Theileria equi in horses raised in the Jaboticabal microregion, Sao Paulo State, Brazil. Rev. Bras. Parasitol. Vet. 2010, 19, 228–232, doi:10.1590/s1984-29612010000400007.
- Kumar, S.; Kumar, R.; Gupta, A.K.; Yadav, S.C.; Goyal, S.K.; Khurana, S.K.; Singh, R.K. Development of EMA-2 recombinant antigen based enzyme-linked immunosorbent assay for seroprevalence studies of Theileria equi infection in Indian equine population. Vet. Parasitol. 2013, 198, 10–17, doi:10.1016/j.vetpar.2013.08.030.
- Kumar, S.; Kumar, Y.; Malhotra, D.V.; Dhar, S.; Nichani, A.K. Standardisation and comparison of serial dilution and single dilution enzyme linked immunosorbent assay (ELISA) using different antigenic preparations of the Babesia (Theileria) equi parasite. Vet. Res. 2003, 34, 71–83, doi:10.1051/vetres:2002055.
- Kappmeyer, L.S.; Perryman, L.E.; Hines, S.A.; Baszler, T.V.; Katz, J.B.; Hennager, S.G.; Knowles, D.P. Detection of equine antibodies to Babesia caballi by recombinant B. caballi rhoptry-associated protein 1 in a competitive-inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1999, 37, 2285–2290, doi:10.1128/JCM.37.7.2285-2290.1999.
- Baldani, C.D.; Hilario, E.; Nakaghi, A.C.; Bertolini, M.C.; Machado, R.Z. Production of recombinant EMA-1 protein and its application for the diagnosis of Theileria equi using an enzyme immunoassay in horses from Sao Paulo State, Brazil. Rev. Bras. Parasitol. Vet. 2011, 20, 54–60, doi:10.1590/s1984-29612011000100011.
- Wang, P.; Song, J.; Song, R.; Zhang, M.; Wu, L.; Li, F.; Yan, Y.; Zhou, J.; Chahan, B.; Liao, M. Preparation of monoclonal antibodies against Bc48 and development of a rapid detection assay for infection with Babesia caballi in China. Folia Parasitol. 2019, 66, doi:10.14411/fp.2019.005.
- Zhang, Y.; Zhang, Y.T.; Wang, Z.B.; Bolati; Li, H.; Bayinchahan. A Duplex PCR Method for Detection of Babesia caballi and Theileria equi. Chin. J. Parasitol. Parasit. Dis. 2015, 33, 105–109.
- Bhoora, R.; Quan, M.; Zweygarth, E.; Guthrie, A.J.; Prinsloo, S.A.; Collins, N.E. Sequence heterogeneity in the gene encoding the rhoptry-associated protein-1 (RAP-1) of Babesia caballi isolates from South Africa. Vet. Parasitol. 2010, 169, 279–288, doi:10.1016/j.vetpar.2010.01.009.
- Mahmoud, M.S.; El-Ezz, N.T.; Abdel-Shafy, S.; Nassar, S.A.; El Namaky, A.H.; Khalil, W.K.; Knowles, D.; Kappmeyer, L.; Silva, M.G.; Suarez, C.E. Assessment of Theileria equi and Babesia caballi infections in equine populations in Egypt by molecular, serological and hematological approaches. Parasites Vectors 2016, 9, 260, doi:10.1186/s13071-016-1539-9.
- Rapoport, A.; Aharonson-Raz, K.; Berlin, D.; Tal, S.; Gottlieb, Y.; Klement, E.; Steinman, A. Molecular characterization of the Babesia caballi rap-1 gene and epidemiological survey in horses in Israel. Infect. Genet. Evol. 2014, 23, 115–120, doi:10.1016/j.meegid.2014.01.033.
- Alhassan, A.; Govind, Y.; Tam, N.T.; Thekisoe, O.M.; Yokoyama, N.; Inoue, N.; Igarashi, I. Comparative evaluation of the sensitivity of LAMP, PCR and in vitro culture methods for the diagnosis of equine piroplasmosis. Parasitol. Res. 2007, 100, 1165–1168, doi:10.1007/s00436-006-0430-6.
- Alhassan, A.; Iseki, H.; Kim, C.; Yokoyama, N.; Igarashi, I. Comparison of polymerase chain reaction methods for the detection of Theileria equi infection using whole blood compared with pre-extracted DNA samples as PCR templates. Trop. Anim. Health Prod. 2007, 39, 369–374, doi:10.1007/s11250-007-9025-1.
- Alhassan, A.; Pumidonming, W.; Okamura, M.; Hirata, H.; Battsetseg, B.; Fujisaki, K.; Yokoyama, N.; Igarashi, I. Development of a single-round and multiplex PCR method for the simultaneous detection of Babesia caballi and Babesia equi in horse blood. Vet. Parasitol. 2005, 129, 43–49, doi:10.1016/j.vetpar.2004.12.018.
- Alhassan, A.; Thekisoe, O.M.; Yokoyama, N.; Inoue, N.; Motloang, M.Y.; Mbati, P.A.; Yin, H.; Katayama, Y.; Anzai, T.; Sugimoto, C.; et al. Development of loop-mediated isothermal amplification (LAMP) method for diagnosis of equine piroplasmosis. Vet. Parasitol. 2007, 143, 155–160, doi:10.1016/j.vetpar.2006.08.014.
- Bhoora, R.V.; Pienaar, R.; Cornelius, F.; Josemans, A.; Matthee, O.; Marumo, R.; Troskie, C.; Mans, B.J. Multiplex hydrolysis-probe assay for the simultaneous detection of Theileria equi and Babesia caballi infections in equids. Vet. Parasitol. 2018, 255, 61–68, doi:10.1016/j.vetpar.2018.03.022.
- Montes Cortes, M.G.; Fernandez-Garcia, J.L.; Habela Martinez-Estellez, M.A. A multinested PCR for detection of the equine piroplasmids Babesia caballi and Theileria equi. Tick Tick-Borne Dis. 2019, 10, 305–313, doi:10.1016/j.ttbdis.2018.11.008.
- Xie, J.; Liu, G.; Tian, Z.; Luo, J. Development of loop-mediated isothermal amplification (LAMP) for detection of Theileria equi. Acta Trop. 2013, 127, 245–250, doi:10.1016/j.actatropica.2013.05.007.
- Salim, B.; Bakheit, M.A.; Sugimoto, C. Rapid detection and identification of Theileria equi and Babesia caballi by high-resolution melting (HRM) analysis. Parasitol. Res. 2013, 112, 3883–3886, doi:10.1007/s00436-013-3581-2.
- Nicolaiewsky, T.B.; Richter, M.F.; Lunge, V.R.; Cunha, C.W.; Delagostin, O.; Ikuta, N.; Fonseca, A.S.; da Silva, S.S.; Ozaki, L.S. Detection of Babesia equi (Laveran, 1901) by nested polymerase chain reaction. Vet. Parasitol. 2001, 101, 9–21, doi:10.1016/s0304-4017(01)00471-x.
- Bhoora, R.; Quan, M.; Franssen, L.; Butler, C.M.; van der Kolk, J.H.; Guthrie, A.J.; Zweygarth, E.; Jongejan, F.; Collins, N.E. Development and evaluation of real-time PCR assays for the quantitative detection of Babesia caballi and Theileria equi infections in horses from South Africa. Vet. Parasitol. 2010, 168, 201–211, doi:10.1016/j.vetpar.2009.11.011.
- Bhoora, R.; Quan, M.; Matjila, P.T.; Zweygarth, E.; Guthrie, A.J.; Collins, N.E. Sequence heterogeneity in the equi merozoite antigen gene (ema-1) of Theileria equi and development of an ema-1-specific TaqMan MGB assay for the detection of T. equi. Vet. Parasitol. 2010, 172, 33–45, doi:10.1016/j.vetpar.2010.04.025.
- Ueti, M.W.; Palmer, G.H.; Kappmeyer, L.S.; Scoles, G.A.; Knowles, D.P. Expression of equi merozoite antigen 2 during development of Babesia equi in the midgut and salivary gland of the vector tick Boophilus microplus. J. Clin. Microbiol. 2003, 41, 5803–5809, doi:10.1128/jcm.41.12.5803-5809.2003.
- Lobanov, V.A.; Peckle, M.; Massard, C.L.; Brad Scandrett, W.; Gajadhar, A.A. Development and validation of a duplex real-time PCR assay for the diagnosis of equine piroplasmosis. Parasites Vectors 2018, 11, 125, doi:10.1186/s13071-018-2751-6.
- Alanazi, A.D.; Said, A.E.; Morin-Adeline, V.; Alyousif, M.S.; Slapeta, J. Quantitative PCR detection of Theileria equi using laboratory workflows to detect asymptomatic persistently infected horses. Vet. Parasitol. 2014, 206, 138–145, doi:10.1016/j.vetpar.2014.09.019.
- Kim, C.M.; Blanco, L.B.; Alhassan, A.; Iseki, H.; Yokoyama, N.; Xuan, X.; Igarashi, I. Diagnostic real-time PCR assay for the quantitative detection of Theileria equi from equine blood samples. Vet. Parasitol. 2008, 151, 158–163, doi:10.1016/j.vetpar.2007.10.023.
- Bhoora, R.; Franssen, L.; Oosthuizen, M.C.; Guthrie, A.J.; Zweygarth, E.; Penzhorn, B.L.; Jongejan, F.; Collins, N.E. Sequence heterogeneity in the 18S rRNA gene within Theileria equi and Babesia caballi from horses in South Africa. Vet. Parasitol. 2009, 159, 112–120, doi:10.1016/j.vetpar.2008.10.004.
- Gubbels, J.M.; de Vos, A.P.; van der Weide, M.; Viseras, J.; Schouls, L.M.; de Vries, E.; Jongejan, F. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J. Clin. Microbiol. 1999, 37, 1782–1789, doi:10.1128/JCM.37.6.1782-1789.1999.
- Bhoora, R.V.; Collins, N.E.; Schnittger, L.; Troskie, C.; Marumo, R.; Labuschagne, K.; Smith, R.M.; Dalton, D.L.; Mbizeni, S. Molecular genotyping and epidemiology of equine piroplasmids in South Africa. Tick Tick-Borne Dis. 2020, 11, 101358, doi:10.1016/j.ttbdis.2019.101358.
- Guidi, E.; Pradier, S.; Lebert, I.; Leblond, A. Piroplasmosis in an endemic area: Analysis of the risk factors and their implications in the control of Theileriosis and Babesiosis in horses. Parasitol. Res. 2015, 114, 71–83, doi:10.1007/s00436-014-4161-9.
- Ruegg, S.R.; Heinzmann, D.; Barbour, A.D.; Torgerson, P.R. Estimation of the transmission dynamics of Theileria equi and Babesia caballi in horses. Parasitology 2008, 135, 555–565, doi:10.1017/S0031182008004204.
- Tirosh-Levy, S.; Gottlieb, Y.; Mazuz, M.L.; Savisky, I.; Steinman, A. Infection dynamics of Theileria equi in carrier horses is associated with management and tick exposure. Tick Tick-Borne Dis. 2020, 101508, doi:10.1016/j.ttbdis.2020.101508.
- Coultous, R.M.; Phipps, P.; Dalley, C.; Lewis, J.; Hammond, T.A.; Shiels, B.R.; Weir, W.; Sutton, D.G.M. Equine piroplasmosis status in the UK: An assessment of laboratory diagnostic submissions and techniques. Vet. Rec. 2019, 184, 95, doi:10.1136/vr.104855.
- Baptista, C.; Lopes, M.S.; Tavares, A.C.; Rojer, H.; Kappmeyer, L.; Mendonca, D.; da Camara Machado, A. Diagnosis of Theileria equi infections in horses in the Azores using cELISA and nested PCR. Tick Tick-Borne Dis. 2013, 4, 242–245, doi:10.1016/j.ttbdis.2012.11.008.
- Adaszek, L.; Gorna, M.; Krzysiak, M.; Adaszek, M.; Garbal, M.; Winiarczyk, S. Identification of the piroplasms isolated from horses with clinical piroplasmosis in Poland. Wiad. Parazytol. 2011, 57, 21–26.
- Short, M.A.; Clark, C.K.; Harvey, J.W.; Wenzlow, N.; Hawkins, I.K.; Allred, D.R.; Knowles, D.P.; Corn, J.L.; Grause, J.F.; Hennager, S.G.; et al. Outbreak of equine piroplasmosis in Florida. J. Am. Vet. Med. Assoc. 2012, 240, 588–595, doi:10.2460/javma.240.5.588.
- Beard, L.A.; Pelzel, A.M.; Rush, B.R.; Wright, A.M.; Galgut, B.I.; Hennager, S.G.; King, A.O.; Traub-Dargatz, J.L. Babesia equi-induced anemia in a Quarter Horse and subsequent regulatory response. J. Am. Vet. Med. Assoc. 2013, 242, 992–996, doi:10.2460/javma.242.7.992.
- Butler, C.M.; van Gils, J.A.; van der Kolk, J.H. A literature review of equine piroplasmosis after an episode of acute babesiosis in a Dutch Standardbred foal after a stay in Normandy. Tijdschr. Voor Diergeneeskd. 2005, 130, 726–731.
- Manna, G.; Cersini, A.; Nardini, R.; Bartolome Del Pino, L.E.; Antognetti, V.; Zini, M.; Conti, R.; Lorenzetti, R.; Veneziano, V.; Autorino, G.L.; et al. Genetic diversity of Theileria equi and Babesia caballi infecting horses of Central-Southern Italy and preliminary results of its correlation with clinical and serological status. Tick Tick-Borne Dis. 2018, 9, 1212–1220, doi:10.1016/j.ttbdis.2018.05.005.
- Ionita, M.; Nicorescu, I.M.; Pfister, K.; Mitrea, I.L. Parasitological and molecular diagnostic of a clinical Babesia caballi outbreak in Southern Romania. Parasitol. Res. 2018, 117, 2333–2339, doi:10.1007/s00436-018-5899-2.
- Tirosh-Levy, S.; Steinman, A.; Levy, H.; Katz, Y.; Shtilman, M.; Gottlieb, Y. Parasite load and genotype are associated with clinical outcome of piroplasm-infected equines in Israel. Parasites Vectors 2020, 13, 267, doi:10.1186/s13071-020-04133-y.
- Camino, E.; Dorrego, A.; Carvajal, K.A.; Buendia-Andres, A.; de Juan, L.; Dominguez, L.; Cruz-Lopez, F. Serological, molecular and hematological diagnosis in horses with clinical suspicion of equine piroplasmosis: Pooling strengths. Vet. Parasitol. 2019, 275, 108928, doi:10.1016/j.vetpar.2019.108928.
- Mehlhorn, H.; Schein, E. Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein 1998. Parasitol. Res. 1998, 84, 467–475, doi:10.1007/s004360050431.
- Sumbria, D.; das Singla, L.; Sharma, A. Theileria equi and Babesia caballi infection of equids in Punjab, India: A serological and molecular survey. Trop. Anim. Health Prod. 2016, 48, 45–52, doi:10.1007/s11250-015-0917-1.
- Costa, S.C.L.; Freitas, J.S.; Silva, A.N.D.; Lacerda, L.C.; Cruz, R.D.S.; Carvalho, F.S.; Pereira, M.J.S.; Munhoz, A.D. Frequency and factors associated with Theileria equi, Babesia caballi and Trypanosoma evansi in equids from Bahia (Northeast Brazil). Rev. Bras. Parasitol. Vet. 2019, 28, 47–58, doi:10.1590/S1984-296120180090.
- Minervino, A.H.H.; Torres, A.C.; Moreira, T.R.; Vinholte, B.P.; Sampaio, B.M.; Bianchi, D.; Portela, J.M.; Sarturi, C.; Marcili, A.; Barreto Junior, R.A.; et al. Factors associated with the prevalence of antibodies against Theileria equi in equids of Western Para, Brazil. Transbound. Emerg. Dis. 2020, 67, 100-105, doi:10.1111/tbed.13268.
- Adaszek, L.; Garcia-Bocanegra, I.; Arenas-Montes, A.; Carbonero, A.; Arenas, A.; Winiarczyk, S. Identification of piroplasms isolated from asymptomatic equine species from southern Spain. Berliner Und Munchener Tierarztliche Wochenschrift 2012, 125, 509–512.
- Aziz, K.J.; Al-Barwary, L.T.O. Epidemiological Study of Equine Piroplasmosis (Theileria equi and Babesia caballi) by Microscopic Examination and Competitive-ELISA in Erbil Province North-Iraq. Iran. J. Parasitol. 2019, 14, 404–412.
- Piantedosi, D.; D’Alessio, N.; Di Loria, A.; Di Prisco, F.; Mariani, U.; Neola, B.; Santoro, M.; Montagnaro, S.; Capelli, G.; Veneziano, V. Seroprevalence and risk factors associated with Babesia caballi and Theileria equi infections in donkeys from Southern Italy. Vet. J. 2014, 202, 578–582, doi:10.1016/j.tvjl.2014.09.025.
- Tirosh-Levy, S.; Gottlieb, Y.; Arieli, O.; Mazuz, M.L.; King, R.; Horowitz, I.; Steinman, A. Genetic characteristics of Theileria equi in zebras, wild and domestic donkeys in Israel and the Palestinian Authority. Tick Tick-Borne Dis. 2019, doi:10.1016/j.ttbdis.2019.101286.
- Oduori, D.O.; Onyango, S.C.; Kimari, J.N.; MacLeod, E.T. A field survey for the seroprevalence of Theileria equi and Babesia caballi in donkeys from Nuu Division, Kenya. Tick Tick-Borne Dis. 2015, 6, 683–688, doi:10.1016/j.ttbdis.2015.05.015.
- Afridi, M.J.K.; Mian, A.H.; Saqib, M.; Abbas, G.; Ali, J.; Mansoor, M.K.; Sial, A.U.R.; Rasheed, I.; Hussain, M.H. Seroprevalence and Risk Factors for Theileria equi Infection in Equines from Khyber Pakhtunkhwa Province, Pakistan. Iran. J. Parasitol. 2017, 12, 597–605.
- Kumar, S.; Kumar, R.; Sugimoto, C. A perspective on Theileria equi infections in donkeys. Jpn. J. Vet. Res. 2009, 56, 171–180.
- Abedi, V.; Razmi, G.; Seifi, H.; Naghibi, A. Molecular detection of equine piroplasms in donkeys (Equus asinus) in North Khorasan province, Iran. Iran. J. Vet. Res. 2015, 16, 202–204.
- Veronesi, F.; Morganti, G.; Ravagnan, S.; Laus, F.; Spaterna, A.; Diaferia, M.; Moretti, A.; Fioretti, D.P.; Capelli, G. Molecular and serological detection of tick-borne pathogens in donkeys (Equus asinus) in Italy. Vet. Microbiol. 2014, 173, 348–354, doi:10.1016/j.vetmic.2014.08.017.
- Hawkins, E.; Kock, R.; McKeever, D.; Gakuya, F.; Musyoki, C.; Chege, S.M.; Mutinda, M.; Kariuki, E.; Davidson, Z.; Low, B.; et al. Prevalence of Theileria equi and Babesia caballi as well as the identification of associated ticks in sympatric Grevy’s zebras (Equus grevyi) and donkeys (Equus africanus asinus) in northern Kenya. J. Wildl. Dis. 2015, 51, 137–147, doi:10.7589/2013-11-316.
- Smith, R.M.; Bhoora, R.V.; Kotze, A.; Grobler, J.P.; Lee Dalton, D. Translocation a potential corridor for equine piroplasms in Cape mountain zebra (Equus zebra zebra). Int. J. Parasitol. Parasites Wildl. 2019, 9, 130–133, doi:10.1016/j.ijppaw.2019.04.010.
- Bhoora, R.; Buss, P.; Guthrie, A.J.; Penzhorn, B.L.; Collins, N.E. Genetic diversity of piroplasms in plains zebra (Equus quagga burchellii) and Cape mountain zebra (Equus zebra zebra) in South Africa. Vet. Parasitol. 2010, 174, 145–149, doi:10.1016/j.vetpar.2010.08.014.
- Lampen, F.; Bhoora, R.; Collins, N.E.; Penzhorn, B.L. Putative clinical piroplasmosis in a Burchell’s zebra (Equus quagga burchelli). J. S. Afr. Vet. Assoc. 2009, 80, 257–260, doi:10.4102/jsava.v80i4.223.
- King’ori, E.M.; Obanda, V.; Ndambiri, E.M.; Runo, S.M.; Chiyo, P.I. Adding injury to infection: The relationship between injury status and genetic diversity of Theileria infecting plains zebra, Equus quagga. Infect. Genet. Evol. 2018, 58, 269–278, doi:10.1016/j.meegid.2018.01.010.
- Zweygarth, E.; Lopez-Rebollar, L.M.; Meyer, P. In vitro isolation of equine piroplasms derived from Cape Mountain zebra (Equus zebra zebra) in South Africa. Onderstepoort J. Vet. Res. 2002, 69, 197–200.
- Li, J.; Li, Y.; Moumouni, P.F.A.; Lee, S.H.; Galon, E.M.; Tumwebaze, M.A.; Yang, H.; Huercha; Liu, M.; Guo, H.; et al. First description of Coxiella burnetii and Rickettsia spp. infection and molecular detection of piroplasma co-infecting horses in Xinjiang Uygur Autonomous Region, China. Parasitol. Int. 2020, 76, 102028, doi:10.1016/j.parint.2019.102028.
- Beck, R.; Vojta, L.; Mrljak, V.; Marinculic, A.; Beck, A.; Zivicnjak, T.; Caccio, S.M. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int. J. Parasitol. 2009, 39, 843–848, doi:10.1016/j.ijpara.2008.12.005.
- Inacio, E.L.; Perez-Macchi, S.; Alabi, A.; Bittencourt, P.; Muller, A. Prevalence and molecular characterization of piroplasmids in domestic dogs from Paraguay. Tick Tick-Borne Dis. 2019, 10, 321–327, doi:10.1016/j.ttbdis.2018.11.009.
- Adamu, M.; Troskie, M.; Oshadu, D.O.; Malatji, D.P.; Penzhorn, B.L.; Matjila, P.T. Occurrence of tick-transmitted pathogens in dogs in Jos, Plateau State, Nigeria. Parasites Vectors 2014, 7, 119, doi:10.1186/1756-3305-7-119.
- Qablan, M.A.; Kubelova, M.; Siroky, P.; Modry, D.; Amr, Z.S. Stray dogs of northern Jordan as reservoirs of ticks and tick-borne hemopathogens. Parasitol. Res. 2012, 111, 301–307, doi:10.1007/s00436-012-2839-4.
- Salim, B.; Alanazi, A.D.; Omori, R.; Alyousif, M.S.; Alanazi, I.O.; Katakura, K.; Nakao, R. Potential role of dogs as sentinels and reservoirs for piroplasms infecting equine and cattle in Riyadh City, Saudi Arabia. Acta Trop. 2019, 193, 78–83, doi:10.1016/j.actatropica.2019.02.029.
- Qablan, M.A.; Sloboda, M.; Jirku, M.; Obornik, M.; Dwairi, S.; Amr, Z.S.; Horin, P.; Lukes, J.; Modry, D. Quest for the piroplasms in camels: Identification of Theileria equi and Babesia caballi in Jordanian dromedaries by PCR. Vet. Parasitol. 2012, 186, 456–460, doi:10.1016/j.vetpar.2011.11.070.
- Bahrami, S.; Tabandeh, M.R.; Tafreshi, A.R.G. Prevalence and Molecular Identification of Piroplasmids in Iranian Dromedaries (Camelus dromedarius). J. Zoo Wildl. Med. 2017, 48, 1026–1030, doi:10.1638/2016-0233.1.
- Sadeddine, R.; Diarra, A.Z.; Laroche, M.; Mediannikov, O.; Righi, S.; Benakhla, A.; Dahmana, H.; Raoult, D.; Parola, P. Molecular identification of protozoal and bacterial organisms in domestic animals and their infesting ticks from north-eastern Algeria. Tick Tick-Borne Dis. 2020, 11, 101330, doi:10.1016/j.ttbdis.2019.101330.
- Da Silveira, A.W.; de Oliveira, G.G.; Menezes Santos, L.; da Silva Azuaga, L.B.; Macedo Coutinho, C.R.; Echeverria, J.T.; Antunes, T.R.; do Nascimento Ramos, C.A.; Izabel de Souza, A. Natural Infection of the South American Tapir (Tapirus terrestris) by Theileria equi. J. Wildl. Dis. 2017, 53, 411–413, doi:10.7589/2016-06-149.
- Laveran, A. Contribution a l’etude de Piroplasma equi. CR Soc. Biol. 1901, 12, 385–388.
- Allsopp, M.T.; Allsopp, B.A. Molecular sequence evidence for the reclassification of some Babesia species. Ann. N. Y. Acad. Sci. 2006, 1081, 509–517.
- Hikosaka, K.; Watanabe, Y.; Tsuji, N.; Kita, K.; Kishine, H.; Arisue, N.; Palacpac, N.M.; Kawazu, S.; Sawai, H.; Horii, T.; et al. Divergence of the mitochondrial genome structure in the apicomplexan parasites, Babesia and Theileria. Mol. Biol. Evol. 2010, 27, 1107–1116, doi:10.1093/molbev/msp320.
- Kappmeyer, L.S.; Thiagarajan, M.; Herndon, D.R.; Ramsay, J.D.; Caler, E.; Djikeng, A.; Gillespie, J.J.; Lau, A.O.; Roalson, E.H.; Silva, J.C.; et al. Comparative genomic analysis and phylogenetic position of Theileria equi. BMC Genom. 2012, 13, 603, doi:10.1186/1471-2164-13-603.
- Lack, J.B.; Reichard, M.V.; van Den Bussche, R.A. Phylogeny and evolution of the Piroplasmida as inferred from 18S rRNA sequences. Int. J. Parasitol. 2012, 42, 353–363, doi:10.1016/j.ijpara.2012.02.005.
- Dahmana, H.; Amanzougaghene, N.; Davoust, B.; Normand, T.; Carette, O.; Demoncheaux, J.P.; Mulot, B.; Fabrizy, B.; Scandola, P.; Chik, M.; et al. Great diversity of Piroplasmida in Equidae in Africa and Europe, including potential new species. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100332, doi:10.1016/j.vprsr.2019.100332.
- Nagore, D.; Garcia-Sanmartin, J.; Garcia-Perez, A.L.; Juste, R.A.; Hurtado, A. Detection and identification of equine Theileria and Babesia species by reverse line blotting: Epidemiological survey and phylogenetic analysis. Vet. Parasitol. 2004, 123, 41–54, doi:10.1016/j.vetpar.2004.04.010.
- Githaka, N.; Konnai, S.; Bishop, R.; Odongo, D.; Lekolool, I.; Kariuki, E.; Gakuya, F.; Kamau, L.; Isezaki, M.; Murata, S.; et al. Identification and sequence characterization of novel Theileria genotypes from the waterbuck (Kobus defassa) in a Theileria parva-endemic area in Kenya. Vet. Parasitol. 2014, 202, 180–193, doi:10.1016/j.vetpar.2014.02.056.
- Caccio, S.; Camma, C.; Onuma, M.; Severini, C. The beta-tubulin gene of Babesia and Theileria parasites is an informative marker for species discrimination. Int. J. Parasitol. 2000, 30, 1181–1185, doi:10.1016/s0020-7519(00)00105-3.
- Wise, L.N.; Kappmeyer, L.S.; Knowles, D.P.; White, S.N. Evolution and diversity of the EMA families of the divergent equid parasites, Theileria equi and T. haneyi. Infect. Genet. Evol. 2019, 68, 153–160, doi:10.1016/j.meegid.2018.12.020.
- Knowles, D.P.; Kappmeyer, L.S.; Perryman, L.E. Genetic and biochemical analysis of erythrocyte-stage surface antigens belonging to a family of highly conserved proteins of Babesia equi and Theileria species. Mol. Biochem Parasitol. 1997, 90, 69–79, doi:10.1016/s0166-6851(97)00150-3.
- Munkhjargal, T.; Sivakumar, T.; Battsetseg, B.; Nyamjargal, T.; Aboulaila, M.; Purevtseren, B.; Bayarsaikhan, D.; Byambaa, B.; Terkawi, M.A.; Yokoyama, N.; et al. Prevalence and genetic diversity of equine piroplasms in Tov province, Mongolia. Infect. Genet. Evol. 2013, 16, 178–185, doi:10.1016/j.meegid.2013.02.005.
- Wang, J.; Liu, J.; Yang, J.; Wang, X.; Li, Z.; Jianlin, X.; Li, X.; Xiang, Q.; Li, Y.; Liu, Z.; et al. The first molecular detection and genetic diversity of Babesia caballi and Theileria equi in horses of Gansu province, China. Tick Tick-Borne Dis. 2019, 10, 528–532, doi:10.1016/j.ttbdis.2019.01.003.
- Hall, C.M.; Busch, J.D.; Scoles, G.A.; Palma-Cagle, K.A.; Ueti, M.W.; Kappmeyer, L.S.; Wagner, D.M. Genetic characterization of Theileria equi infecting horses in North America: Evidence for a limited source of U.S. introductions. Parasites Vectors 2013, 6, 35, doi:10.1186/1756-3305-6-35.
- Ketter-Ratzon, D.; Tirosh-Levy, S.; Nachum-Biala, Y.; Saar, T.; Qura’n, L.; Zivotofsky, D.; Abdeen, Z.; Baneth, G.; Steinman, A. Characterization of Theileria equi genotypes in horses in Israel, the Palestinian Authority and Jordan. Tick Tick-Borne Dis. 2017, 8, 499–505, doi:10.1016/j.ttbdis.2017.02.010.
- Ruegg, S.R.; Torgerson, P.; Deplazes, P.; Mathis, A. Age-dependent dynamics of Theileria equi and Babesia caballi infections in southwest Mongolia based on IFAT and/or PCR prevalence data from domestic horses and ticks. Parasitology 2007, 134, 939–947, doi:10.1017/S0031182007002405.
- Heim, A.; Passos, L.M.; Ribeiro, M.F.; Costa-Junior, L.M.; Bastos, C.V.; Cabral, D.D.; Hirzmann, J.; Pfister, K. Detection and molecular characterization of Babesia caballi and Theileria equi isolates from endemic areas of Brazil. Parasitol. Res. 2007, 102, 63–68, doi:10.1007/s00436-007-0726-1.
- Seo, M.G.; Yun, S.H.; Choi, S.K.; Cho, G.J.; Park, Y.S.; Cho, K.H.; Kwon, O.D.; Kwak, D. Molecular and phylogenetic analysis of equine piroplasms in the Republic of Korea. Res. Vet. Sci. 2013, 94, 579–583, doi:10.1016/j.rvsc.2013.01.014.
- Salim, B.; Bakheit, M.A.; Kamau, J.; Nakamura, I.; Sugimoto, C. Nucleotide sequence heterogeneity in the small subunit ribosomal RNA gene within Theileria equi from horses in Sudan. Parasitol. Res. 2010, 106, 493–498, doi:10.1007/s00436-009-1691-7.
- Bishop, R.P.; Kappmeyer, L.S.; Onzere, C.K.; Odongo, D.O.; Githaka, N.; Sears, K.P.; Knowles, D.P.; Fry, L.M. Equid infective Theileria cluster in distinct 18S rRNA gene clades comprising multiple taxa with unusually broad mammalian host ranges. Parasites Vectors 2020, 13, 261, doi:10.1186/s13071-020-04131-0.
- Tirosh-Levy, S.; Steinman, A.; Einhorn, A.; Apanaskevich, D.A.; Mumcuoglu, K.Y.; Gottlieb, Y. Potential tick vectors for Theileria equi in Israel. Med. Vet. Entomol. 2020, doi:10.1111/mve.12435.
- Coultous, R.M.; McDonald, M.; Raftery, A.G.; Shiels, B.R.; Sutton, D.G.M.; Weir, W. Analysis of Theileria equi diversity in The Gambia using a novel genotyping method. Transbound. Emerg. Dis. 2019, 10.1111/tbed.13454, doi:10.1111/tbed.13454.