The spontaneous evolution of colorectal cancer is always burdened by complications. The most common complication is low bowel obstruction, found in approximately 20% of the cases of colorectal cancer, and it can occur either relatively abruptly, or is preceded by initially discrete premonitory symptoms, non-specific (until advanced evolutionary stages) and generally neglected or incorrectly interpreted. Success in the complex treatment of a low neoplastic obstruction is conditioned by a complete diagnosis, adequate pre-operative preparation, a surgical act adapted to the case (in one, two or three successive stages), and dynamic postoperative care. The moment of surgery should be chosen with great care and is the result of the experience of the anesthetic-surgical team. The operative act must be adapted to the case and has as its main objective the resolution of intestinal obstruction and only in a secondary way the resolution of the generating disease.
- cancer
- colorectal
- obstruction
1. Introduction
2. Development of the Obstructive Mechanism
2.1. Anatomical Factors
2.1.1. The Diameter of the Colorectal Segments
2.1.2. The Thickness of the Colic Wall
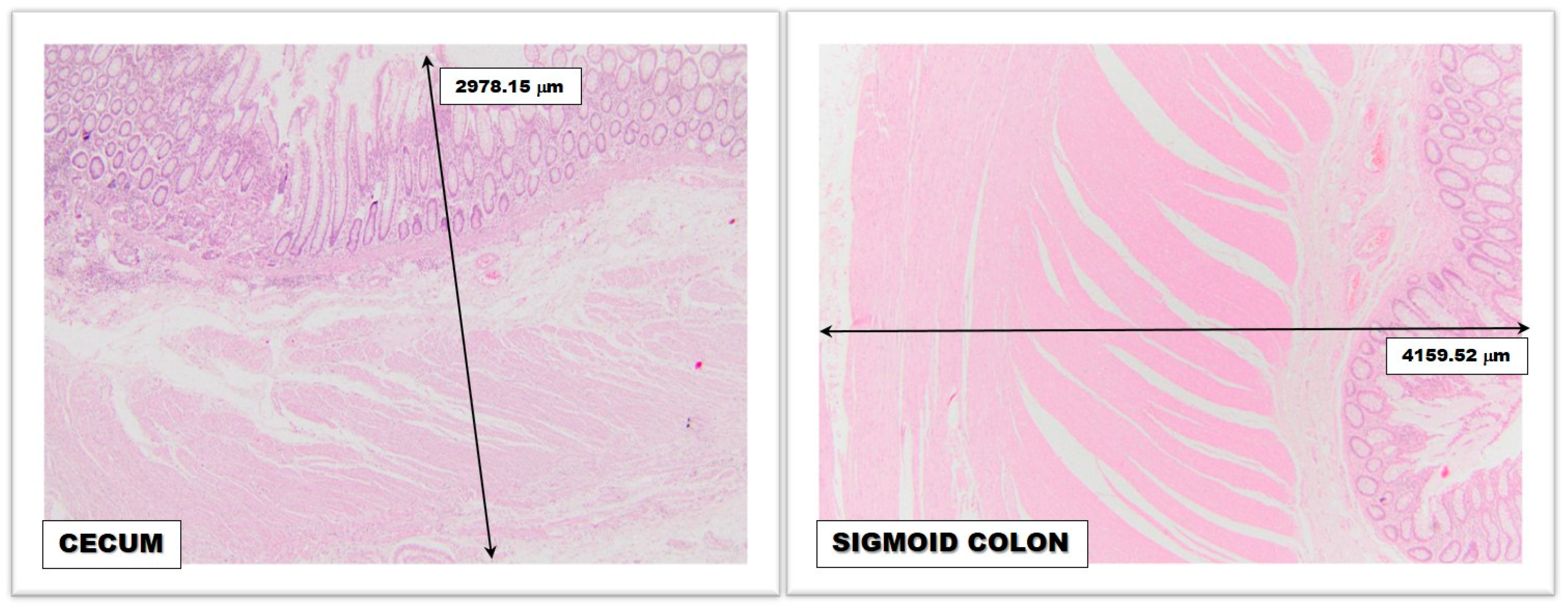
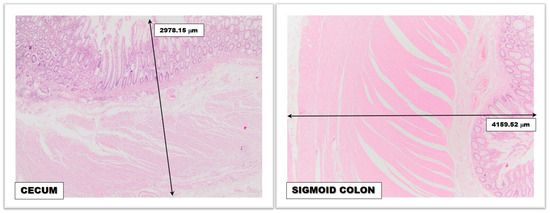
2.1.3. Colorectal Vascularization
2.1.4. Anatomical and Functional Sphincters
2.1.5. The Mobility of Colic Segments
2.1.6. Tumor Morphopathology
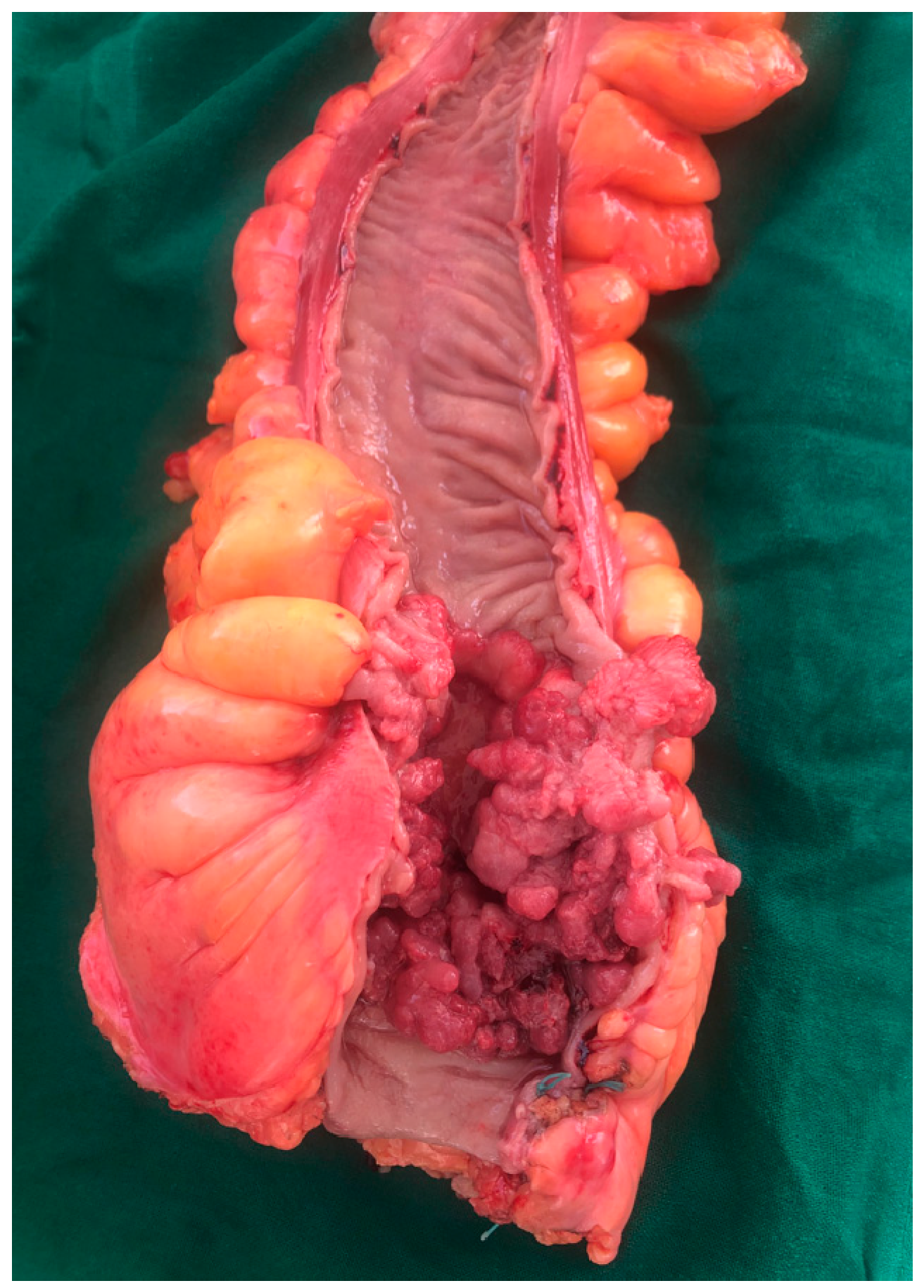
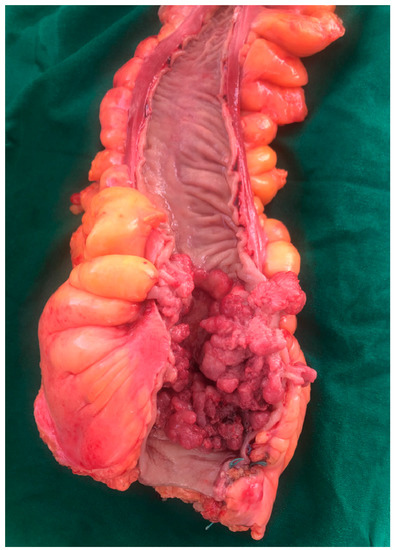
2.2. Functional Factors
2.2.1. Colic Peristalsis
2.2.2. Other Functional Factors
References
- Bancu, S. Bowel obstructions. In Surgical Textbook; Popescu, I., Ed.; Romanian Academy Publishing: Bucharest, Romania, 2008; p. 1094.
- Koşar, M.N.; Görgülü, Ö. Incidence and mortality results of intestinal obstruction in geriatric and adult patients: 10 years retrospective analysis. Turk. J. Surg. 2021, 37, 363–370.
- Yang, X.-F.; Pan, K. Diagnosis and management of acute complications in patients with colon cancer: Bleeding, obstruction, and perforation. Chin. J. Cancer Res. 2014, 26, 331–340.
- Kirchhoff, P.; Clavien, P.-A.; Hahnloser, D. Complications in colorectal surgery: Risk factors and preventive strategies. Patient Saf. Surg. 2010, 4, 5.
- Webster, P.J.; Aldoori, J.; Burke, D.A. Optimal management of malignant left-sided large bowel obstruction: Do international guidelines agree? World J. Emerg. Surg. 2019, 14, 1–8.
- Roeland, E.; Gunten, C.F. Current concepts in malignant bowel obstruction management. Curr. Oncol. Rep. 2009, 11, 298–303.
- Machicado Zuñiga, E.; Giraldo Casas, R.C.; Fernández, K.F.E.; Geng Cahuayme, A.A.A.; García Dumler, D.; Fernández Concha Llona, I.; Fisher Alvarez, M.; Cano Córdova, A.S. Localización y clínica asociada al cáncer de colon: Hospital Nacional Arzobispo Loayza: 2009–2013. Horiz. Méd. 2015, 15, 49–55.
- Winner, M.; Mooney, S.J.; Hershman, D.L.; Feingold, D.L.; Allendorf, J.D.; Wright, J.D.; Neugut, A.I. Incidence and Predictors of Bowel Obstruction in Elderly Patients with Stage IV Colon Cancer: A Population-Based Cohort Study. JAMA Surg. 2013, 148, 715–722.
- Demb, J.; Earles, A.; Martínez, M.E.; Bustamante, R.; Bryant, A.K.; Murphy, J.D.; Liu, L.; Gupta, S. Risk factors for colorectal cancer significantly vary by anatomic site. BMJ Open Gastroenterol. 2019, 6, e000313.
- Gainant, A. Emergency management of acute colonic cancer obstruction. J. Visc. Surg. 2012, 149, e3–e10.
- Rubin, J.; Principe, D.R.; Movitz, B.; Ng, M.; Kochar, K. Cecum perforation secondary to plunger-induced barotrauma. J. Surg. Case Rep. 2019, 2019, rjz077.
- Pouli, S.; Kozana, A.; Papakitsou, I.; Daskalogiannaki, M.; Raissaki, M. Gastrointestinal perforation: Clinical and MDCT clues for identification of aetiology. Insights Into Imaging 2020, 11, 1–19.
- Shao, J.; Sun, L.; Fu, Q. Small Bowel Perforation Due to Blunt Trauma of Left Leg with an Incarcerated Inguinal Hernia: A Case Report. Front. Surg. 2021, 8, 710417.
- Baer, C.; Menon, R.; Bastawrous, S.; Bastawrous, A. Emergency Presentations of Colorectal Cancer. Surg. Clin. N. Am. 2017, 97, 529–545.
- Wang, S.C.; Schulman-Marcus, J.; Fantauzzi, J.; Bevington, T.; Sayegh, A.; Lee, E.; Ata, A.; Kambam, M.; Sidhu, M.; Lyubarova, R. Colon cancer laterality is associated with atherosclerosis and coronary artery disease. J. Gastrointest. Oncol. 2019, 10, 30–36.
- Yamaji, Y.; Yasunaga, H.; Hirata, Y.; Yamada, A.; Yoshida, S.; Horiguchi, H.; Fushimi, K.; Koike, K. Association Between Colorectal Cancer and Atherosclerotic Diseases: A Study Using a National Inpatient Database in Japan. Dig. Dis. Sci. 2016, 61, 1677–1685.
- Gagliardi, J.A.; Radvany, M.G.; Kilkenny, T.E.; Russo, R.D. Colonic sphincters revisited: Simulators of organic disease. Hawaii Med. J. 1994, 53, 278–282.
- McKnight, S.T.; Myers, A.; Canon, C.L.; Hawn, M. A functional colonic obstruction: Cannon’s point. Radiol. Case Rep. 2011, 6, 557.
- McCullough, J.; Engledow, A. Treatment Options in Obstructed Left-sided Colonic Cancer. Clin. Oncol. 2010, 22, 764–770.
- Marzouk, O.; Schofield, J. Review of Histopathological and Molecular Prognostic Features in Colorectal Cancer. Cancers 2011, 3, 2767–2810.
- Duan, H.; Cai, X.; Luan, Y.; Yang, S.; Yang, J.; Dong, H.; Zeng, H.; Shao, L. Regulation of the Autonomic Nervous System on Intestine. Front. Physiol. 2021, 12, 700129.
- Lin, Y.-M.; Fu, Y.; Winston, J.; Radhakrishnan, R.; Sarna, S.K.; Huang, L.-Y.M.; Shi, X.-Z. Pathogenesis of abdominal pain in bowel obstruction: Role of mechanical stress-induced upregulation of nerve growth factor in gut smooth muscle cells. Pain 2017, 158, 583–592.
- Artemev, A.; Naik, S.; Pougno, A.; Honnavar, P.; Shanbhag, N.M. The Association of Microbiome Dysbiosis with Colorectal Cancer. Cureus 2022, 14, e22156.
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Qin, H. Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. 2015, 6, 20.
- Chia-Hui Yu, L. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: Exploring a common ground hypothesis. J. Biomed. Sci. 2018, 25, 79.
- Hegde, S.; Lin, Y.-M.; Golovko, G.; Khanipov, K.; Cong, Y.; Savidge, T.; Fofanov, Y.; Shi, X.-Z. Microbiota dysbiosis and its pathophysiological significance in bowel obstruction. Sci. Rep. 2018, 8, 1–12.
- Stefano Corazziari, E. Intestinal mucus barrier in normal and inflamed colon. J. Pediatr. Gastroenterol. Nutr. 2009, 48, S54–S55.
- Pisano, M.; Zorcolo, L.; Merli, C.; Cimbanassi, S.; Poiasina, E.; Ceresoli, M.; Agresta, F.; Allievi, N.; Bellanova, G.; Coccolini, F.; et al. 2017 WSES guidelines on colon and rectal cancer emergencies: Obstruction and perforation. World J. Emerg. Surg. 2018, 13, 1–27.
- Yoo, R.-N.; Cho, H.-M.; Kye, B.-H. Management of obstructive colon cancer: Current status, obstacles, and future directions. World J. Gastrointest. Oncol. 2021, 13, 1850–1862.
- Adler, D.G. Management of malignant colonic obstruction. Curr. Treat. Options Gastroenterol. 2005, 8, 231–237.
- Soybel, D.; Santos, A. Ileus and bowel obstruction. In Greenfield’s Surgery. Scientific Principles and Practice, 6th ed.; Mulholland, M., Lillemoe, K., Doherty, G., Upchurch, G., Jr., Alam, H., Pawlik, T., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2017; pp. 782–808.
- Stoyanov, H.; Julianov, A.; Valtchev, D.; Matev, A. Results of the treatment of colorectal cancer complicated by obstruction. Wien. Klin. Wochenschr. 1998, 110, 262–265.
- Fabrizio, A.; Wick, E. The Management of Large Bowel Obstruction. In Current Surgical Therapy, 12th ed.; Cameron, J., Cameron, A., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 180–182.
- Angelescu, N. Bowel Obstructions; Surgical Textbook; Editura Medicala: Bucharest, Romania, 2003; pp. 2168–2184.
