Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Marta Woldanska-Okonska.
Osteoarthritis (OA) is a common disease among the human population worldwide. OA causes functional impairment, leads to disability and poses serious socioeconomic burden. The rehabilitation offers a function-oriented method to reduce the disability using diverse interventions (kinesiotherapy, physical therapy, occupational therapy, education, and pharmacotherapy).
- osteoarthrosis
- rehabilitation
- kinesiotherapy
- physical therapy
- inflammation regulation
1. Kinesiotherapy-Treatment with Movement
Since the dawn of rehabilitation as a medical discipline after two world wars, kinesiotherapy plays a central role in rehabilitation [24][1]. As a consequence of accepting the analogy between training and drugs, kinesiotherapy demands appropriate dosage and form [25][2]. It also determines contraindications and side effects of kinesiotherapy. The training has a great impact on homeostasis and the intra-articular environment and a vast number of articles proving these effects have been published.
The meta-analysis by Alves et al. showed evident and significant changes in the chemokine profile after long distance running [26][3]. The authors concluded that this exhausting form of sport activity up-regulated proinflammatory IL-6, IL-8, TNF-α serum concentration and caused a parallel increase in the concentration of anti-inflammatory factors IL-1RA (Il-1 receptor antagonist) and IL-10. Long distance running also decreased the IFN-γ and leptin concentration. The changes were more strongly expressed in marathon and ultra-marathon competitors. The research shows the complexity of effort-induced reactions since the final anti-inflammatory or proinflammatory effects are not clear. The training increased the concentration of cartilage oligomeric matrix protein as well as a cartilage matrix damage markers. Balan et al. compared young and old sedentary males with young and old trained cyclists. The results showed positive effects of training on inflammatory markers (Il-8, TNFα) but a lack of significant impact on senescence markers in muscle tissues [27][4]. This research proves that the tissue senescence does not depend strictly on inflammation (in muscle tissues at least). Nakamura et al. [28][5] examined a murine model of cartilage destruction. The obtained results suggested that a joint surface load reduction activates cartilage degeneration by NF-κB signaling. The NF-κB consists of several proteins that activate the cell response to stress factors such as cytokines, reactive oxygen species or antigens. An inactive NF-κB is present in cytosol. The activation may occur via classical (dependent on e.g., TNFα, IL-1β, T-cell receptors) or alternative pathways (triggered by adaptive immunity factors). The dysregulation of NF-κB in OA, rheumatoid arthritis and tumor genesis was observed [29][6]. Jimi et al. suggested that both significantly low and excessively high NF-κB activation leads to osteoarthritis (OA) progression [30][7]. Deficient NF-κB activity promotes apoptosis, whereas high activity increases inflammation, up-regulation of matrix metalloproteinases and vascular endothelial growth factor (VEGF). The exercise increases the reactive oxygen species (ROS) and reactive nitrogen species (RNS) present in the joints and plasma [31][8]. The RNS in joints are mainly produced by chondrocytes and macrophages present in large numbers in the synovial membrane [32][9]. The ROS and RNS, as particles with high oxidative potential, take part in inflammatory reactions and destroy the tissues, e.g., the chondral matrix. On the other hand, the exercise enhances the antioxidative mechanisms. The ROS and RNS activate mitogen-activated kinases (MAPKs), which reorganize the muscle cells genetic activity essential to training-induced adaptive mechanisms [31][8]. The above-mentioned mechanisms are presented in Figure 1. The data suggests the training should ensure moderate intensity as the exhaustion disturbs the balance and activates destructive catabolic processes including chondrocyte senescence and protease activation [33][10].
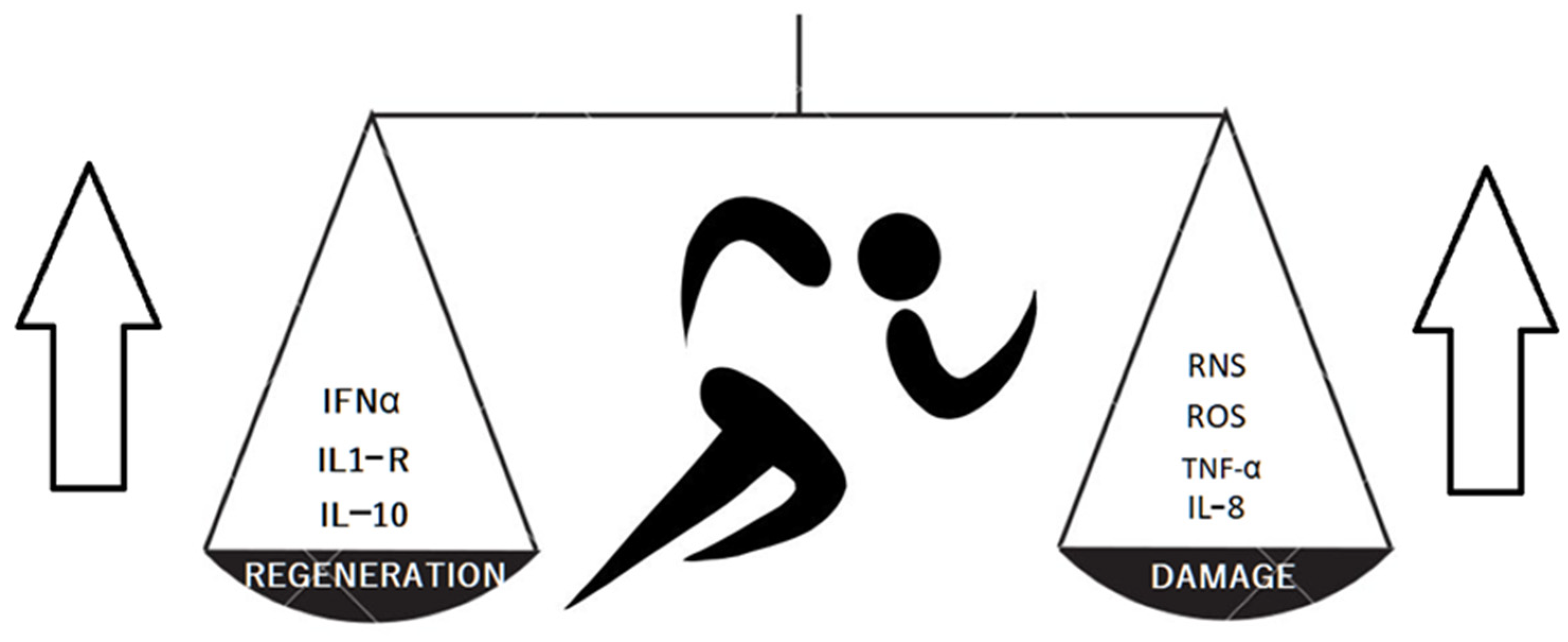
Figure 1.
The training results in producing both cartilage protective and damaging factors.
The treadmill exercise shows beneficial effects on inflammation by inhibiting spleen secretion of TNFα, IL-6 and corticosterone increase. The data shows a positive exercise effect on the whole body by general anti-inflammatory action [34][11]. The review by Kong et al. recapitulates the role of exercise in main molecular processes [31][8].
1.1. Non-Coding RNA
Non-coding RNA (ncRNA) represents a group of RNA not translated into proteins. There are many types of ncRNA including short ncRNA: microRNA (miRNA ranging from 17 to 25 nt), small interfering RNAs (siRNAs) and piwi-interacting RNAs (piRNAs), as well as long non-coding RNA (lncRNA longer than 200-nucleotides). These studies discovered that ncRNA plays a major role in signaling and cellular regulation mechanisms [35][12].
1.2. Micro RNA (miRNA)
The micro RNA was discovered in the 1990s, primarily in procaryotic organisms. Through the decades, these small RNA fragments with different nucleotide sequences were identified and their regulative role became clear. There are more than 50 diseases and developmental disturbances associated with the altered expression of miRNA [36][13]. Surprisingly, the absence of some miRNA particles causes a better health state (e.g., improved glucose tolerance). miRNA modulates gene expression via guiding Argonaute (AGO) proteins to specific target RNAs which leads in consequence to either RNA degradation or translational repression [37][14].
Endisha et al. revealed that miR-34a-5p overexpression causes increased cartilage damage, promotes chondrocyte apoptosis and results in clinical deterioration. The miR-34-5p knockdown reduces these negative effects [37][14]. Ito et al. reported a protective role of miR-455-5p and miR-455-3p on cartilage; these particles hampered hypoxia-inducible factor-2α activity HIF2α (on a mouse model) [38][15]. HIF2α expression in the cell was induced by low oxygen concentration. HIF2α is activated by creating a complex with sitruin-1 and plays a vital role in activating angiogenesis processes. The miR-455-5p and miR-455-3p synthesis depends on Sox9—the transcription factor responsible for transforming mesenchymal cells into chondrocytes and stimulating chondrocytes normal activity including chondral matrix reparation [39][16]. The low Sox9 activity in the late stage of OA was observed. Carbonare et al. [40][17] investigated the impact of half-marathon training on mesenchymal circulating progenitor cells. The authors found that the training increased the Sox9 activity, which resulted in the transformation of progenitors into chondrocytes.
Ding et al. confirmed that miRNA-93 reduced inflammation and protected chondrocytes from apoptosis [41][18] via interaction with Toll-Like Receptor 4 and preventing NF-κB activation. The protective role of miR146a in osteoarthrosis was characterized by Guan et al. [42][19]. While working on both human and mice models, the authors discovered that miR146a inhibited the degeneration of cartilage by inactivating Notch 1. The action took place in both post-traumatic and primary OA. Notch 1 is a transmembrane protein influencing cell differentiation and cell communication with other closely located cells. Notch1 presence on chondrocytes located on the cartilage surface was observed and its overexpression characterized chondrocytes in OA. MiR146a has a complementary binding site to Notch1. Notch 1-miR146a interaction leads to down-regulation of Il-1β, IL6, TNF α and attenuates the IL-1β destructive procatabolic activity [42][19]. Minguzzi et al. reported a reduction in metalloproteinase MMP1, MMP3 and MMP10 expression by Notch1 knockdown, another mechanism of cartilage protection [43][20]. The results confirmed earlier observations on Notch1 role in OA. At the moment there are no results of research concerning the impact of training on miR-455-3p and miR-455-5p expression.
Horak et al. described the significant changes in extracellular levels of extracellular miR-16, miR-21, miR-93 and miR-222 after performing different forms of training over eight weeks. The volunteers were young male athletic students. The collected data showed changes in circulating RNA (up- or down-regulation). The results are confusing as the four studied miRNAs have a vast influence on many metabolic pathways and receptors [44][21].
Wardle et al. found differences in plasma miR-16, miR-21, miR-93 and miR-222 levels between the group of endurance-trained and strength-trained athletes [45][22]. The authors emphasized the sports medicine aspect of the research and did not link the results with OA. In 2021, Stadnik et al. described the regulative role of miR222 (miR-221, miR-21 and miR-27) in cartilage exposed to excessive compression [46][23]. The increased level of above-mentioned molecules up-regulated tissue inhibitors of metalloproteinase 3 (TIMP3) and cytoplasmic polyadenylation element binding protein-3 (CPEB3). Metalloproteinase 3 cleaves the aggrecan-cartilage matrix basic proteoglycan binding hyaluronic acid and ensures proper cartilage matrix hydration [47][24]. Thus, the destruction of aggrecan affects the cartilage matrix architecture and function promoting OA development. CPEB3 dysregulation often accompanies occurrence of neoplasm originating from different organs [48][25]. It is worth mentioning that large compressing forces activate chondral degradation in obese patients.
The circular RNA (circRNA) are commonly present in eucaryotic cells. The circRNA has the C-3 and C-5 endings connected by a covalent bond, which makes it resistant to exonuclease-dependent degradation [48][25]. Nowadays, the regulative role of circRNA in osteoarthritis has been widely investigated. There have been many circRNA molecules proven to have an important regulative function in aging process regulation (e.g., in Alzheimer disease, heart failure and renal fibrosis) in humans and other organisms. Salzman et al. found that the circRNA sequences represented cell type specificity [49][26]. Circ_0043947 and circ_0000205 expression increases the presence of Il-1 β and both induce apoptosis and cartilage matrix destruction, however both of these molecules activate different pathways [50,51][27][28]. The research suggests that the knockdown of these circRNA may protect the cartilage and reduce the destructive Il-1 β effect.
2. Physical Therapy
Physical therapy uses multiple forms of physical energies, e.g., electric current, magnetic field, mechanical waves, infrared radiation, short and microwaves and tissue cooling (cryotherapy). According to international rehabilitation and physiotherapy societies, guidelines and publications, the physical therapy role in general is being reduced, especially in western countries [82][29]. There are many profound differences on dosage, duration and other parameters of applied physical stimuli. In different countries, multiple parameters and devices are used which makes performing meta-analyses impossible. Some forms of physical therapy are examined in the context of joint molecular environment.
3. Ultrasound Therapy
Ultrasound therapy (US) uses (in rehabilitation physical treatment) devices producing ultrasound wave with power ranging from 0.05 mW/cm2 up to 2 W/cm2. The frequency applied usually ranges from 0.8 MHz to 3 MHz and the wave energy absorption depth ranges from 1 cm (3 MHz frequency) to 3 cm (0.8 MHz frequency) [83][30]. In OA the ultrasound wave application takes place in an affected area (the wave penetrates the joint). The ultrasound wave application in some cases forms impulses, in others the wave constantly penetrates the tissue [84][31]. Among multiple physical therapy methods the ultrasound therapy draws scientific attention, whereas other popular forms of physical treatment (e.g., transcutaneous electric nerve stimulation-TENS and treatment with local and general body cooling-cryotherapy) lack scientifically-proven efficacy and molecular background studies in OA [85,86][32][33].
Chinese scientists examined the low intensity pulsed ultrasound (LIPUS) influence on mouse chondrocytes. The results showed the inhibition of proarthrotic vascular endothelium growth factor A (VEGFA) expression which delayed the cartilage loss. LIPUS therapy (average intensity of 30 mW·cm−2, frequency of 1.5 MHz, pulse repetition rate of 1 kHz and the on–off ratio of 20% for 20 min) caused the up-regulation of p38 mitogen-activated protein kinase inhibitor [87][34]. The research on rabbits with surgically triggered knee OA treated with US showed significant changes in synovial fluid protein after US application (continuous mode, 1.5 W/cm2, 3 MHz, 10 min per session and five times per week, for two weeks) [88][35]. Observed changes in proteome had a protective effect, however further investigation should help uresearchers understand the whole mechanism of the above-mentioned procedure. Xia et al. examined the LIPUS application (50 mW/cm2, on–off ratio of 20%, frequency of 3 MHz) on OA rats chondrocyte co-culture and observed positive effect of ultrasound on mesenchymal stem cell differentiation in cartilage. The authors suggested that LIPUS promotes autophagy (cell self-digestion) with exosome release. Scientists suggest exosome release is essential in transforming mesenchymal cells into chondrocytes [89][36]. The positive results of long-lasting LIPUS therapy applied on patients with moderate knee arthrosis (4 h daily with 0.13 W/cm2 power intensity) on pain reduction and functional improvement were reported by Draper et al. [90][37]. As low intensity ultrasound does not produce any perceptible for patient sensations, the authors succeeded in performing a placebo-controlled double blind study. This clinical trial does not show the molecular background of the LIPUS effect.
4. Magnetic Field Therapy
Therapy with a magnetic field has a long history in physical treatment. Magnetic field influences all the tissues generating electric polarization. The mechanisms underlying magnetic field therapy effects remains unclear, however biophysics and quantum biology show wide a range of possible and proven effects [91][38]. The magnetic field therapy shows a variety of forms (constant and pulse therapy, magneto-stimulation with μT induction values and 100–1000 Hz basal frequency, magnetotherapy with 1–10 mT induction values and 1–3000 Hz frequency) and various effects as well [92][39].
Parate et al. used magnetotherapy (10 min 15 Hz impulses amplitude 0.4–5 mT) to investigate its impact on cartilage and mesenchymal stem cells in vitro [93][40]. The therapy showed a positive effect increase in mesenchymal stem cells transforming into chondrocytes and apoptosis and inflammation inhibition as well. The up-regulation of cell growth factors and anti-inflammatory cytokines (Il-7, Il-11, macrophage colony stimulating factor M-CSF, stem cell factor SCF) was described in the review by Hamid et al. [94][41]. The cell growth factors (FGF-fibroblast growth factor, TGF-β1 transforming growth factor β1, cell cycle regulator c-Jun) are released intensely as magnetic field increase free K+ and Ca2+ ions. These changes have a strong effect on cells by enhancing mitotic division and protein synthesis; the anti-inflammatory regenerative processes take advantage. Magnetic field exposure has immunomodulatory properties and the macrophage functional change (anti-inflammatory M2 or proinflammatory M1) depends on applied parameters [95][42]. The positive effect of pulsed magnetic field was concluded by Yang et al. in a meta-analysis study [92][39] confirming the positive effect of pulsed electromagnetic field therapy used for OA treatment on pain reduction, stiffness and physical function. The coherent results were presented by Tong et al. [96][43].
5. Laser Therapy
Laser has multiple uses in medicine ranging from surgery, oncology and ophthalmology to rehabilitation. Laser devices are capable of precise dosing electromagnetic wave energy of a certain wavelength. Although laser therapy was invented in the 1960s, its biological activity remains the subject of research until now. In rehabilitation, low level laser therapy (LLLT) with red (wavelength approx. 780 nm) and near infrared (wavelength 808–830 nm) electromagnetic wave application with power output reaching 100 mW [97][44]. Low level laser therapy does not induce thermal effects, however it activates many biological processes. Wound healing, pain reduction and inflammation suppression are the most common indications for LLLT. Nambi concluded the recent studies performed on a rat osteoarthrosis model in a meta-analysis [98][45]. The results showed beneficial effects on cytokine profile the reduction in IL-1β, Il-6, TNFα and MMP-13 decrease. Saygun et al. applied low level laser irradiation in vitro on osteoblasts. The irradiation increased the release of basic fibroblast growth factor (bFGF), Insulin-Like Growth Factor I (IGF-I), IGF-I receptor and up-regulated osteoblast activity [99][46]. However, vast differences in LLLT parameters (wavelength, power density, time of application, energy absorbed) applied prevent reaching some clinical conclusions [100][47]. Akaltun et al. investigated the high intensity laser therapy (HILT) with IR 1064 nm wavelength laser with a maximal power of 12 W as an addition to the exercise session in knee osteoarthritis versus placebo laser with exercise session. The patients received in total 3000 J of laser energy during 10 sessions. The authors confirmed the effectiveness of HILT addition in pain reduction, range of motion improvement and cartilage preservation [101][48]. The positive effect of HILT (wavelength 1064 nm energy applied 9.77 J/cm2) on IL-10 and vascular endothelial growth factor (VEGF) released from equine mesenchymal stem cells was discovered by Peat et al. [102][49].
References
- American Kinesiotherapy Association. Available online: https://akta.org/about/history (accessed on 4 February 2023).
- Przeździak, B. Historia Rehabilitacji na świecie i w Polsce in Rehabilitacja Medyczna; Kwolek, A., Ed.; Elsevier Urban & Partner: Wrocław, Poland, 2012; pp. 4–9.
- Alves, M.D.d.J.; Silva, D.d.S.; Pereira, E.V.M.; Pereira, D.D.; de Sousa Fernandes, M.S.; Santos, D.F.C.; Oliveira, D.P.M.; Vieira-Souza, L.M.; Aidar, F.J.; de Souza, R.F. Changes in Cytokines Concentration Following Long-Distance Running: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 838069.
- Balan, E.; De Groote, E.; Bouillon, M.; Vieconte, N.; Mahieu, M.N.; Aslain, D.; Nielens, H.; Decottignies, A.; Deldicque, L. No effect of the endurance training status on senescence despite reduced inflammation in skeletal muscle of older individuals. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E447–E454.
- Nakamura, Y.; Saitou, M.; Komura, S.; Matsumoto, K.; Ogawa, H.; Miyagawa, T.; Saitou, T.; Imai, Y.; Takayanagi, H.; Akiyama, H. Reduced dynamic loads due to hip dislocation induce acetabular cartilage degeneration by IL-6 and MMP3 via the STAT3/periostin/NF-κB axis. Sci. Rep. 2022, 12, 12207.
- Park, M.H.; Hong, J.T. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15.
- Jimi, E.; Huang, F.; Nakatomi, C. NF-κB Signaling Regulates Physiological and Pathological Chondrogenesis. Int. J. Mol. Sci. 2019, 20, 6275.
- Kong, H.; Wang, X.-Q.; Zhang, X.-A. Exercise for Osteoarthritis: A Literature Review of Pathology and Mechanism. Front. Aging Neurosci. 2022, 14, 854026.
- Di Meo, S.; Napolitano, G.; Venditti, P. Mediators of Physical Activity Protection against ROS-Linked Skeletal Muscle Damage. Int. J. Mol. Sci. 2019, 20, 3024.
- Chen, C.-W.; Chen, C.-C.; Jian, C.-Y.; Lin, P.-H.; Chou, J.-C.; Teng, H.-S.; Hu, S.; Lieu, F.-K.; Wang, P.S.; Wang, S.-W. Attenuation of exercise effect on inflammatory responses via novel role of TLR4/PI3K/Akt signaling in rat splenocytes. J. Appl. Physiol. 2016, 121, 870–877.
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics–challenges and potential solutions. Nat. Rev. 2021, 20, 629–651.
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51.
- Ebert, M.S.; Sharp, P.A. MicroRNA sponges: Progress and possibilities. RNA 2010, 16, 2043–2050.
- Endisha, H.; Datta, P.; Sharma, A.; Nakamura, S.; Rossomacha, E.; Younan, C.; Ali, S.A.; Tavallee, G.; Lively, S.; Potla, P.; et al. MicroRNA-34a5p Promotes Joint Destruction During Osteoarthritis. Arthritis Rheumatol. 2021, 73, 426–439.
- Ito, Y.; Matsuzaki, T.; Ayabe, F.; Mokuda, S.; Kurimoto, R.; Matsushima, T.; Tabata, Y.; Inotsume, M.; Tsutsumi, H.; Liu, L.; et al. Both microRNA-455-5p and -3p repress hypoxia-inducible factor-2α expression and coordinately regulate cartilage homeostasis. Nat. Commun. 2021, 12, 4148.
- Lefevebre, V.; Angelozzi, M.; Haseeb, A. SOX9 in cartilage development and disease. Curr. Opin. Cell Biol. 2019, 61, 39–47.
- Carbonare, L.D.; Mottes, M.; Cheri, S.; Deiana, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Salvagno, G.L.; Lippi, G.; Valenti, M.T. Increased Gene Expression of RUNX2 and SOX9 in Mesenchymal Circulating Progenitors Is Associated with Autophagy during Physical Activity. Oxid. Med. Cell Longev. 2019, 2019, 8456259.
- Ding, Y.; Wang, L.; Zhao, Q.; Wu, Z.; Kong, L. MicroRNA-93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NF-κB signaling pathway. Int. J. Mol. Med. 2019, 42, 779–790.
- Guan, Y.-J.; Li, J.; Yang, X.U.; Du, S.; Ding, J.; Gao, Y.; Zhang, Y.; Yang, K.; Chen, Q. Evidence that miR-146a attenuates aging and trauma induced osteoarthritis by inhibiting Notch1, IL-6, and IL-1 mediated catabolism. Aging Cell 2018, 17, e12752.
- Minguzzi, M.; Panichi, V.; D’Adamo, S.; Cetrullo, S.; Cattini, L.; Flamigni, F.; Mariani, E.; Borzì, R.M. Pleiotropic Roles of NOTCH1 Signaling in the Loss of Maturational Arrest of Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2021, 22, 12012.
- Horak, M.; Zlamal, F.; Iliev, R.; Kucera, J.; Cacek, J.; Svobodova, L.; Hlavonova, Z.; Kalina, T.; Slaby, O.; Bienertova-Vasku, J. Exercise- induced circulating microRNA changes in athletes in various training scenarios. PLoS ONE 2018, 13, e0191060.
- Wardle, S.L.; Bailey, M.E.S.; Kilikevicius, A.; Malkova, D.; Wilson, R.H.; Venckunas, T.; Moran, C.N. Plasma MicroRNA Levels Differ between Endurance and Strength Athletes. PLoS ONE 2015, 10, e0122107.
- Stadnik, P.S.; Gilbert, S.J.; Tarn, J.; Charlton, S.; Skelton, A.J.; Barter, M.J.; Duance, V.C.; Young, D.A.; Blain, E.J. Regulation of microRNA-221, -222, -21 and -27 in articular cartilage subjected to abnormal compressive forces. J. Physiol. 2021, 599, 143–155.
- Wick, M.; Härönen, R.; Mumberg, D.; Bürger, C.; Olsen, B.R.; Budarf, M.L.; Apte, S.S.; Müller, R. Structure of the Human TIMP-3 gene and its cell cycle-regulated promoter. Biochem. J. 1995, 311, 549–554.
- Wilusz, J.E. A360 degree view of circular RNAs; From biogenesis to functions Wiley Interdiscip. Rev. RNA 2018, 9, e1478.
- Salzman, J.; Chen, R.E.; Olsen, M.N.; Wang, P.L.; Brown, P.O. Cell-Type Specific Features of Circular RNA Expression. PLoS Genet. 2013, 9, e1003777.
- He, M.; Jia, Z.; Wen, Y.; Chen, X. Circ_0043947 contributes to interleukin 1β-induced injury in chondrocytes by sponging miR-671-5p to up-regulate RTN3 expression in osteoarthritis pathology. J. Orthop. Surg. Res. 2022, 17, 177.
- Li, G.; Luo, H.; Ding, Z.; Liang, H.; Laio, Z.; Chen, S.; Huang, Y. Silencing of circ_0000205 mitigates interleukin-1β-induced apoptosis and extracellular matrix degradation in chondrocytes via targeting miR-766-3p/ADAMTS5 axis. Innate Immun. 2022, 28, 79–90.
- Bennell, K.L.; Buchbinder, R.; Himan, R.S. Physical therapies in the management of osteoarthritis: Current state of the evidence. Curr. Opin. Rheumatol. 2015, 27, 304–311.
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans. Biomed. Eng. 2019, 66, 2704–2718.
- Miller, D.; Smith, N.; Bailey, M.; Czarnota, G.; Hynynen, K.; Makin, I. Overview of Therapeutic Ultrasound Applications and Safety Considerations. J. Ultrasound. Med. 2012, 31, 623–634.
- Mansouri, V.; Arjmand, B.; Tavirani, M.R.; Razzaghi, M.; Rostami-Nejad, M.; Hamdieh, M. Evaluation of Efficacy of Low-Level Laser Therapy. J. Lasers Med. Sci. 2020, 11, 369–380.
- Johnson, M.I.; Paley, C.A.; Jones, G.; Mulvey, M.R.; Wittkopf, P.G. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: A systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open 2022, 12, e051073.
- Guan, M.; Zhu, Y.; Liao, B.; Tan, Q.; Qi, H.; Zhang, B.; Huang, J.; Du, X.; Bai, D. Low-intensity pulsed ultrasound inhibits VEGFA expression in chondrocytes and protects against cartilage degeneration in experimental osteoarthritis. FEBS Open Bio 2020, 10, 434–443.
- Luo, Q.; Ji, S.; Li, Z.; Huang, T.; Fan, S.; Xi, Q. Effects of ultrasound therapy on the synovial fluid proteome in a rabbit surgery-induced model of knee osteoarthritis. Biomed. Eng. Online 2019, 18, 18.
- Xia, P.; Wang, Q.; Song, J.; Wang, X.; Wang, X.; Lin, Q.; Cheng, K.; Chen, A.; Li, X. Low-Intensity Pulsed Ultrasound Enhances the Efficacy of Bone Marrow–Derived MSCs in Osteoarthritis Cartilage Repair by Regulating Autophagy-Mediated Exosome Release. Cartilage 2022, 13, 1–13.
- Draper, D.O.; Klyve, D.; Ortiz, R.; Best, T.M. Effect of low-intensity long-duration ultrasound on the symptomatic relief of knee osteoarthritis: A randomized, placebo controlled double-blind study. J. Orthop. Surg. Res. 2018, 13, 257.
- Zadeh-Haghighi, H.; Simon, C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J. R. Soc. Interface 2020, 19, 20220325.
- Yang, X.; Hongchen, H.; Wenwen, Y.; Perry, T.A.; He, C. Effects of Pulsed Electromagnetic Field Therapy on Pain, Stiffness, Physical Function, and Quality of Life in Patients with Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Phys. Ther. 2020, 100, 1118–1131.
- Parate, D.; Kadir, N.D.; Celik, C.; Lee, E.H.; Hui, J.P.; Franco-Obregón, A.; Yang, Z. Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration. Curr. Stem. Cell Res. Ther. 2020, 11, 46.
- Hamid, H.A.; Sarmadi, V.H.; Prasad, V.; Ramasay, R.; Miskon, A. Electromagnetic field exposure as a plausible approach to enhance the proliferation and differentiation of mesenchymal stem cells in clinically relevant scenarios. J. Zhejiang Univ. Sci. B 2022, 23, 42–57.
- Lei, H.; Pan, L.; Wu, R.; Lv, Y. Innate Immune Regulation Under Magnetic Fields with Possible Mechanisms and Therapeutic Applications. Front. Immunol. 2020, 11, 582772.
- Tong, J.; Chen, Z.; Sun, G.; Zhou, J.; Zeng, Y.; Zhong, P.; Deng, C.; Chen, X.; Liu, L.; Wang, S.; et al. The Efficacy of Pulsed Electromagnetic Fields on Pain, Stiffness, and Physical Function in Osteoarthritis: A Systematic Review and Meta-Analysis. Pain Res. Manag. 2022, 2022, 9939891.
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533.
- Nambi, G. Does low level laser therapy has effects on inflammatory biomarkers IL-1β, IL-6, TNF-α, and MMP-13 in osteoarthritis of rat models—A systemic review and meta-analysis. Lasers Med. Sci. 2020, 36, 475–484.
- Saygun, I.; Nizam, N.; Ural, A.U.; Serdar, M.A.; Avcu, F.; Tözüm, T.F. Low-Level Laser Irradiation Affects the Release of Basic Fibroblast Growth Factor (bFGF), Insulin-Like Growth Factor-I (IGF-I), and Receptor of IGF-I (IGFBP3) from Osteoblasts Photomed. Laser Surg. 2012, 30, 149–154.
- Reyegani, S.M.; Raeissadat, S.A.; Heidari, S.; Moradi-Joo, M. Safety and Effectiveness of Low-Level Laser Therapy in Patients with Knee Osteoarthritis: A Systematic Review and Meta-analysis. J. Lasers Med. Sci. 2017, 8 (Suppl. 1), S12–S19.
- Akaltun, M.S.; Altindag, O.; Turan, N.; Gursoy, S.; Gur, A. Efficacy of high intensity laser therapy in knee osteoarthritis: A double-blind controlled randomized study. Clin. Rheumatol. 2020, 40, 1989–1995.
- Peat, F.J.; Colbath, A.C.; Bentsen, L.M.; Goodrich, L.R.; King, M.R. In Vitro Effects of High-Intensity Laser Photobiomodulation on Equine Bone Marrow-Derived Mesenchymal Stem Cell Viability and Cytokine Expression. Photomed. Laser Surg. 2017, 36, 83–91.
More
