Transcriptome complexity is emerging as an unprecedented and fascinating domain, especially by high-throughput sequencing technologies that have unveiled a plethora of new non-coding RNA biotypes. Several sense–antisense transcript pairs have been recently annotated, especially from mammalian genomes, and an understanding of their evolutionary sense and functional role for human health and diseases is only beginning. Antisense long non-coding RNAs ((lncRNAs) dysregulation is significantly involved in hepatocarcinogenesis, where they can act as oncogenes or oncosuppressors, thus playing a key role in tumor onset, progression, and chemoradiotherapy response. Mechanistically, antisense lncRNAs regulate gene expression by exploiting various molecular mechanisms shared with other ncRNA molecules, and exploit special mechanisms on their corresponding sense gene due to sequence complementarity, thus exerting epigenetic, transcriptional, post-transcriptional, and translational controls.
- non-coding RNA
- antisense lncRNA
- HCC
- ceRNET
1. Introduction
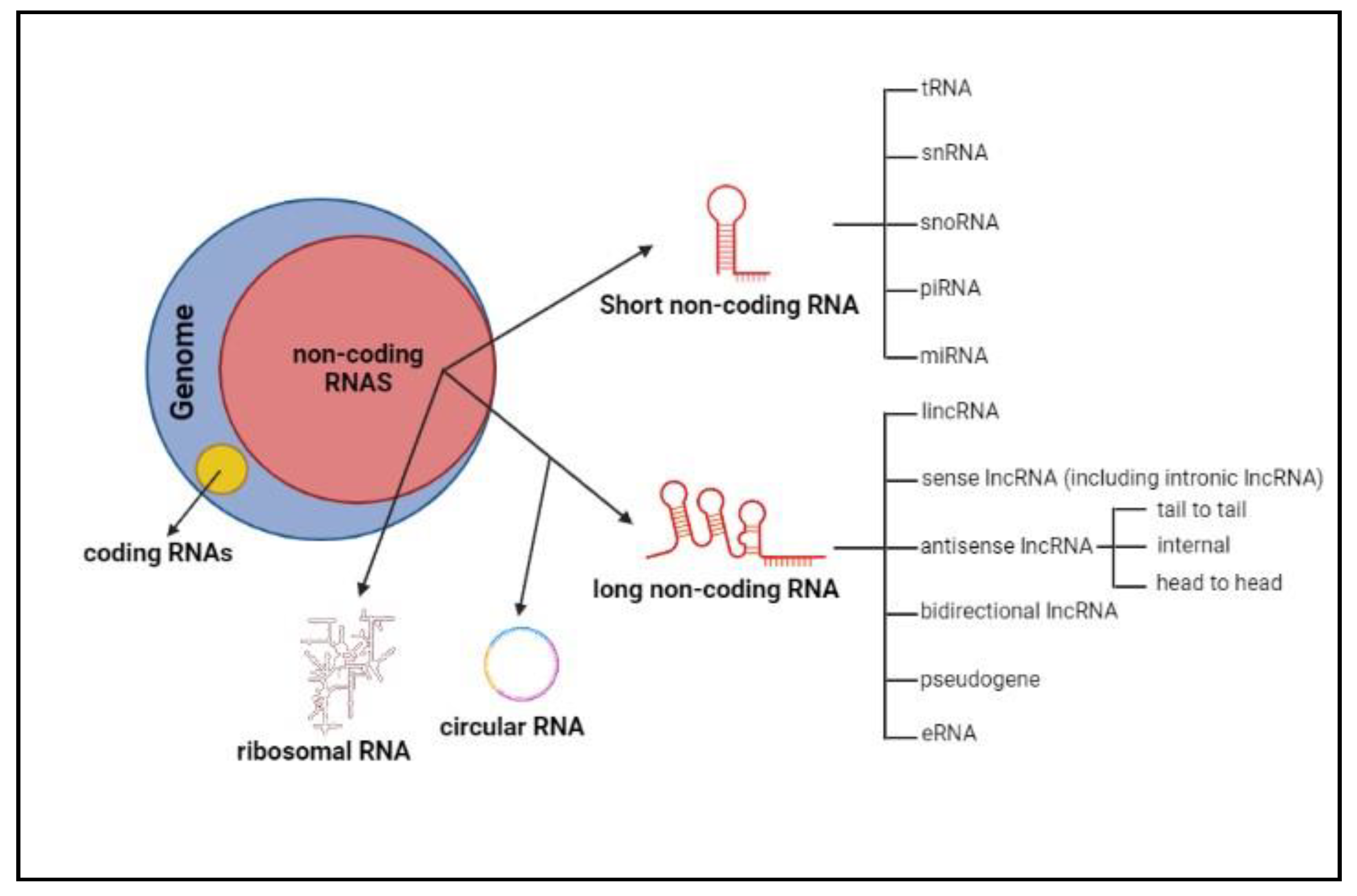
2. Biogenesis and Functioning of Antisense lncRNAs
Antisense lncRNAs and, generally, lncRNA biogenesis, share various features with coding RNAs and precursor transcripts of miRNAs: they are transcribed, generally, by RNA polymerase II, subjected to 5′-capping, 3′-polyadenylation, and splicing, since they are mainly composed of two exons [2]. In contrast with mRNAs that move to the cytoplasm for translation, antisense lncRNAs can be retained in the nucleus. Similarly to coding RNAs, but even more distinctly, lncRNAs exhibit highly specific cell lineage and restricted spatiotemporal and tissue type expression patterns, although they are detected in lower amounts [3]. It is still challenging to assign a mechanism/role to the increasing numbers of annotated antisense lncRNAs, due to their lower amounts and poor evolutionary conservation when compared to coding RNAs. In this regard, it should be noted that conservation may be found in secondary structures rather than in sequences; in fact, a crucial feature of lncRNAs is the ability to form thermodynamically stable structures, a structural versatility enabling them to bind to DNA, other RNA molecules, and proteins [21][22]. In addition, an RNA molecule comprising 100nt can capture more than 5 proteins simultaneously, making RNA molecules a more cost-effective scaffold for protein interaction, in comparison to proteins themselves, with well-known modules/motifs dedicated to interactions [23]. In the nucleus and cytoplasm, antisense lncRNAs exploit all the mechanisms of gene regulation known for other lncRNA biotypes. However, antisense lncRNAs can also reroute these mechanisms onto their sense genes. In addition, due to sequence complementary, antisense lncRNAs can play a special role regarding sense genes (Figure 2). As a consequence, an efficient manner to obtain clues regarding the main mechanism of action of antisense lncRNAs is to detect their prevailing subcellular localization while taking into consideration a possible shuttling between different compartments under specific physiological or pathological conditions. Then, it is possible to speculate that some of the activities performed in that compartment may involve the regulatory contribution of antisense lncRNAs.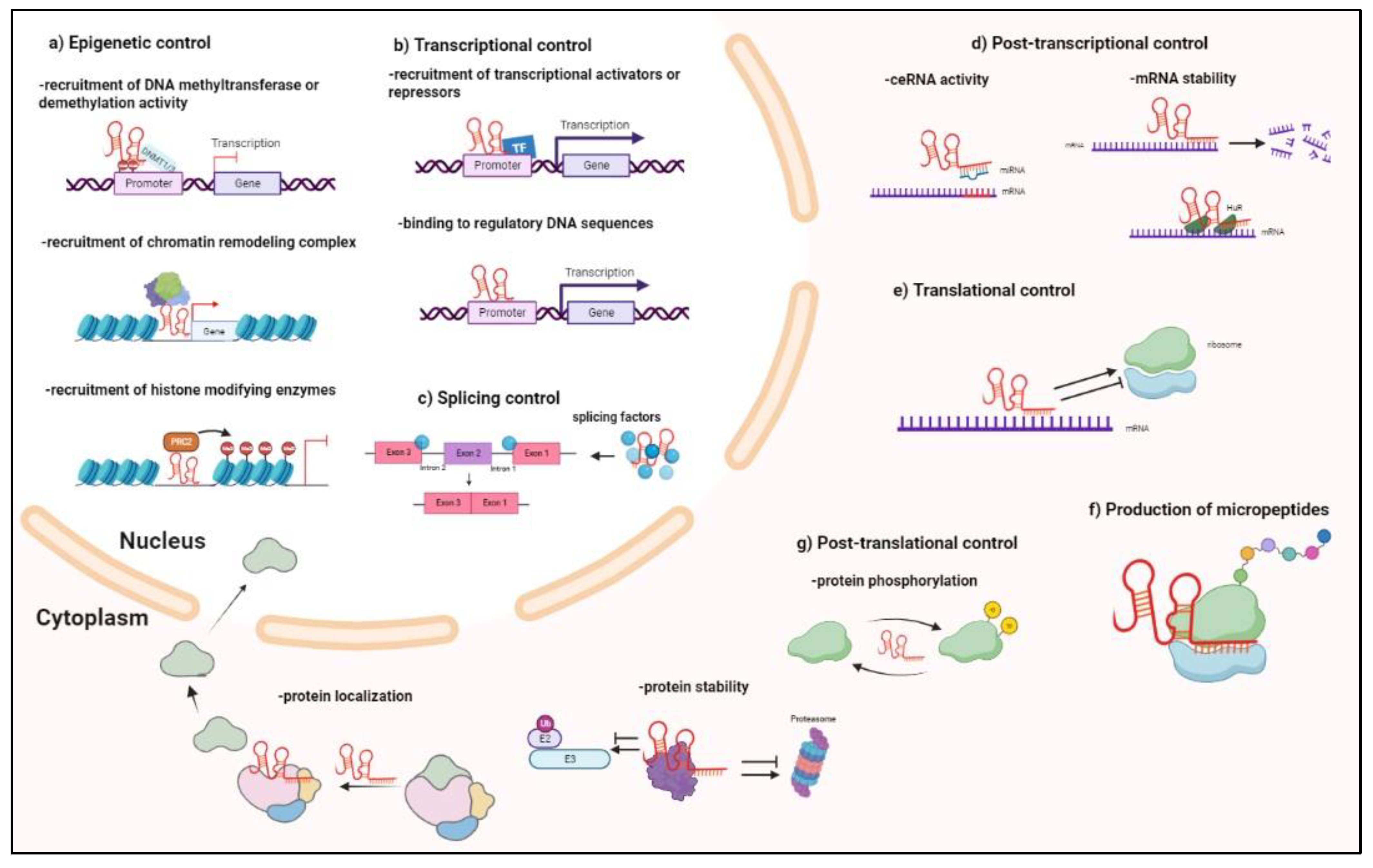
3. Antisense lncRNAs Involved in HCC
Antisense lncRNAs are increasingly recognized as mediators of human cancers [11] and, depending on the context, they can act as either oncogenes or tumor suppressors. In the liver, a number of antisense lncRNAs are described as deregulated, thus playing a crucial role in the onset and progression of HCC [16]. In particular, by searching “(antisense lncRNA) AND (HCC)” throughout PubMed, more than 200 articles were retrieved; they were then analyzed and articles concerning antisense lncRNAs were grouped as detailed below. This analysis was also based on information retrieved from https://lncipedia.org (accessed on 3 April 2023) [46]. An extended list of antisense lncRNAs, their dysregulation, and their molecular functions in HCC is provided in Table 1.|
Antisense lncRNA |
Role |
Effect |
Molecular Mechanism |
Reference |
|---|---|---|---|---|
|
AIRN |
Oncogene |
Promotes proliferation and inhibits apoptosis |
Inhibits CUL4A-mediated ubiquitination of STAT1 |
[47] |
|
ALKBH3-AS1 |
Oncogene |
Promotes cell invasion and proliferation |
Enhances ALKBH3 mRNA stability |
[48] |
|
ANRIL |
Oncogene |
Associated with clinical outcomes; promotes proliferation, migration and invasion; promotes tumor growth and metastasis in vivo; enhances mitochondrial function |
Silences epigenetically Kruppel-like factor 2 (KLF2) by binding to PRC2 sponges let-7c-5p to upregulate NAP1L1, thus activating AKT/mTOR pathway; sponges miR-191, inactivating NF-κB and Wnt/β-catenin pathways; sponges miR-153-5p to upregulate ARHGAP18 and activate MEK/ERK signaling; sponges miR-199a-5p to upregulate ARL2; sponges miR-122-5p |
|
|
BACE1-AS |
Oncogene |
Promotes cell cycle progression, migration, and invasion |
Sponges miR-214-3p to upregulate APLN expression |
[55] |
|
BAIAP2-AS1 |
Oncogene |
Promotes proliferation and metastasis |
Sponges miR-361-3p to release SOX4 |
[56] |
|
BSG-AS1 |
Oncogene |
Correlates to hypoxia; promotes proliferation and migration |
Enhances the stability of BSG mRNA |
[57] |
|
DARS-A1 |
Oncogene |
Correlates with poor prognosis; promotes proliferation, cell invasion, and EMT |
Sponges miR-3200-5p to upregulate CKAP2 and activate the FAK/ERK pathway |
[58] |
|
DDX11-AS1 |
Oncogene |
Promotes proliferation, migration, invasion and glucose metabolism |
Sponges miR-195-5p to upregulate MACC1 expression |
[59] |
|
DLG1-AS1 |
Oncogene |
Promotes proliferation, migration, and invasion in HCC and tumor growth in vivo |
Induced by MYC; sponges miR-497-5p to upregulare SSRP1 |
[60] |
|
DLGAP1-AS1 |
Oncogene |
Promotes proliferation |
Sponges miR-486-5p to upregulate H3F3B |
[61] |
|
DNAJC3-AS1 |
Oncogene |
Correlates with prognosis of patients; promotes proliferation |
Suppresses miR-27b maturation |
[62] |
|
DLX6-AS1 |
Oncogene |
Promotes cell viability, invasion, and migration |
Sponges miR-513c to upregulate Cul4A, thus repressing ANXA10 degradation; sponges miR-424-5p to upregulate WEE1 |
|
|
FAM83H-AS1 |
Oncogene |
Associated with tumor prognosis; promotes proliferation, migration, and invasion |
Inhibits the Wnt/β-catenin pathway by reducing β-catenin and WNT1 expression |
[65] |
|
FGFR3-AS1 |
Oncogene |
Promotes proliferation, migration, and invasion; promotes tumor growth in vivo |
Activates the PI3K/AKT pathway |
[66] |
|
FOXP4-AS1 |
Oncogene |
Associated with poor survival, promotes tumor growth in vivo |
Recruits EZH2 to the promoter region of ZC3H12D to mediate H3K27me3 methylation, thus inhibiting ZC3H12D expression |
[29] |
|
GATA3-AS1 |
Oncogene |
Promotes cell proliferation and metastasis |
Suppresses PTEN, CDKN1A, and TP53 |
[67] |
|
GPC3-AS1 |
Oncogene |
Indicates poor prognosis; proliferation and migration; Promotes xenograft tumor growth in nude mice |
Recruits PCAF to the GPC3 gene body region, upregulating GPC3 transcription |
[68] |
|
HOTAIR |
Oncogene |
Promotes proliferation, migration, invasion, and tumor growth in vivo; regulates the G1/S phase transition; regulates glycolyis; associated with poor survival rates |
Increases ATG3 and ATG7 expression; inhibits RBM38; activates Wnt/β-catenin pathway, increases CCND1 expression and STAT3 signaling; binds STAT3 andP300 to upregulate FUT8 and MUC1; upregulates GLUT1, upregulating mTOR; sponges miR-130a-3p to upregulate HIF1A regulated by FOXC1; sponges miR-1; sponges miR-214-3p to upregulate FLOT1 |
|
|
HOXA11-AS1 |
Oncogene |
Promotes proliferation, invasion, and self-renewal |
Suppresses the transcription of HOXA11 by recruiting DNMT1 to the promoter activating Wnt/βcatenin pathway |
[25] |
|
HOXA-AS2 |
Oncogene |
Promotes cell migration and invasion by inducing EMT |
Sponges miR-520c-3p to upregulate GPC3 |
[77] |
|
HOXD-AS1 |
Oncogene |
Promotes proliferation and invasion; regulates cell cycle progression |
Sponges miR-miR-326 to upregulate SLC27A4, induces MEK/ERK signaling pathway |
|
|
KCNQ1OT1 |
Oncogene |
Correlates with liver cirrhosis, an advanced TNM stage, and a large tumor size; promotes proliferation and tumor growth in vivo |
Sponges miR-504 to regulate GSK3β/β-catenin/Bcl-2 signaling pathway |
[80] |
|
KTN1-AS1 |
Oncogene |
Associated with poor survival, promotes proliferation |
Sponges miR-23c to upregulate ERBB2IP |
[81] |
|
LASP1-AS |
Oncogene |
Associated with poor prognosis, enhances proliferation and migration |
Upregulates LASP1 |
[82] |
|
LEF1-AS1 |
Oncogene |
Promotes proliferation, invasion, angiogenesis, and tumor growth in vivo |
Sponges miR-136-5p to regulate WNK1 espression, recruits CEBPB to promote CDCA7/EZH2 expression |
|
|
LOXL1-AS1 |
Oncogene |
Promotes proliferation, migration, and invasion |
Sponges miR-3614-5p to upregulate YY1 |
[84] |
|
MACC1-AS |
Oncogene |
Increases stemness; promotes cell proliferation, EMT, and invasion |
Sponges miR-145 to regulate Nanog, Oct4, and Sox9; regulates PAX8 |
|
|
MAFG-AS1 |
Oncogene |
Promotes proliferation, invasion, and migration |
Sponges miR-6852 |
[87] |
|
MAPKAPK5-AS1 |
Oncogene |
Associated with poor clinical features and prognosis, promotes growth and metastasis |
Sponges miR-154-5p to upregulate PLAGL2, thus activating EGFR/AKT signaling and regulating HIFA |
[88] |
|
MCM3AP-AS1 |
Oncogene |
Correlated with poor prognosis, promotes cell growth |
Sponges miR-194-5p to upregulate FOXA1 |
[89] |
|
MFI2-AS1 |
Oncogene |
Promotes invasion and metastasis of HCC cells in vitro and vivo |
Sponges miR-134 to upregulate FOXM1 expression |
[90] |
|
MKLN1-AS |
Oncogene |
Promotes proliferation, migration, invasion, and tumor growth in vivo; associated with poor prognosis |
Sponges miR-22-3p to upregulate ETS proto-oncogene 1, sponges miR-654-3p to upregulate HDGF |
|
|
MYLK-AS1 |
Oncogene |
Associated with poor prognosis; promotes cell invasion, migration, proliferation, and angiogenesis |
Sponges miR-424-5p to upregulate E2F7 and activate VEGFR2 signaling; increases EGFR, pEGFR, HER2 and RAF1 expression |
|
|
NNT-AS1 |
Oncogene |
Decreases CD4 lymphocyteinfiltration, promotes proliferation in vitro and tumor growth in vivo |
Enhances TGF-β signaling pathway, sponges miR-363 to upregulate CDK6 expression |
|
|
NPSR1-AS1 |
Oncogene |
Promotes proliferation and glycolysis |
Regulates MAPK/ERK pathway |
[97] |
|
NR2F1-AS1 |
Oncogene |
Induces glycolysis under hypoxia and promotes migration |
Sponges miR-140 to upregulate HK2 |
[98] |
|
OTUD6B-AS1 |
Oncogene |
Promotes proliferation and invasion |
Sponges miR-664b3-p to induce GSKIP/Wnt/β-catenin signalling |
[99] |
|
PCNA-AS1 |
Oncogene |
Promotes tumor growth in vitro and in vivo |
Stabilizes PCNA transcripts |
[100] |
|
PITPNA-AS1 |
Oncogene |
Promotes proliferation, migration, and EMT |
Sponges miR-876-5p to upregulate WNT5A |
[101] |
|
PRKAG2-AS1 |
Oncogene |
Associated with poor survival rates; promotes proliferation, migration, and invasion |
Sponges miR-502-3p to upregulate BICD2 |
[102] |
|
PRR34-AS1 |
Oncogene |
Promotes proliferation migration, invasion, and EMT; enhances tumor growth in vivo |
Sponges miR-296-5p to upregulate E2F2 and SOX12, activating Wnt/βcatenin pathway; interacts with DDX3X to regulate the stability of Rab27a mRNA and promote the exosome secretion of VEGF and TGF-β; sponges miR-498 to upregulate TOMM20 and ITGA6 |
|
|
RBM5-AS1 |
Oncogene |
Promotes cell proliferation and invasion |
Sponges miR-132/212 via recruiting PRC2 complex |
[106] |
|
RHPN1-AS1 |
Oncogene |
Correlated with prognosis of patients; promotes proliferation and metastasis; associated with the occurrence of lymphatic metastasis and a higher level of serum AFP; correlated with poor survival |
STAT1 induces overexpression of RHPN1-AS1, sponges miR-485 to upregulate CDCA5, sponges miR-596 to upregulate IGF2BP2 |
|
|
RNF185-AS1 |
Oncogene |
Correlated with advanced TNM stage, distant metastasis and a poor survival rate; promotes proliferation, migration, and invasion |
Sponges miR-221-5p to upregulate ITGB5 |
[109] |
|
SBF2-AS1 |
Oncogene |
Correlated with poor prognosis, promotes proliferation and tumor growth in vivo |
Sponges miR-140-5p to upregulate TGFBR1 expression |
[110] |
|
SNAI3-AS1 |
Oncogene |
Promotes proliferation and metastasis |
Sponges miR-27-3p/34a-5p |
[111] |
|
SOX9-AS1 |
Oncogene |
Promotes proliferation, migration, and invasion; Promotes tumor growth and metastasis in vivo |
Sponges miR-5590-3p to upregulate SOX9, thus activating Wnt/b-catenin pathway |
[112] |
|
SPACA6P-AS |
Oncogene |
Promotes cell proliferation |
Sponges miR-125a/Let7a to upregulate Lin28b, MMP11, SIRT7, Zbtb7a, Cyclin D1, CDC25B, HMGA2 |
[113] |
|
ST8SIA6-AS1 |
Oncogene |
Promotes proliferation, migration, and invasion |
Sponges miR-338-3p to upregulate NONO expression, sponges miR-5195-3p to regulate HOXB6 expression |
|
|
TMPO-AS1 |
Oncogene |
Associated with poor prognosis, promotes proliferation and EMT |
Sponges miR-126-3p to upregulate LRP6, inducing Wnt/β-catenin signalling; sponges miR-329-3p to upregulate FOXK1, inducing AKT/mTOR signaling pathway; sponges miR-320a to upregulate SERBP1 |
|
|
TP73-AS1 |
Oncogene |
Correlated with poor prognosis, promotes proliferation |
Sponges miR-200a to induce HMGB1/RAGE pathway |
[119] |
|
TRG-AS1 |
Oncogene |
Promotes proliferation, migration, invasion, and EMT progress |
Sponges miR-4500 to modulate BACH1 |
[120] |
|
TRIM52-AS1 |
Oncogene |
Promotes proliferation and EMT |
Sponges miR-514a-5p to upregulate MRPS18A |
[121] |
|
TTN-AS1 |
Oncogene |
Promotes proliferation, migration, and EMT |
Sponges miR-139-5p to upregulate SPOCK1 expression |
[122] |
|
UNC5B-AS1 |
Oncogene |
Promotes proliferation, migration, and EMT |
Sponges miR-4306 to upregulate KDM2A expression |
[123] |
|
UPK1A-AS1 |
Oncogene |
Correlated with poor prognosis, promotes proliferation and cell cycle progression |
Interacts with EZH2; sponges miR-138-5p |
[124] |
|
USP2-AS1 |
Oncogene |
Increases proliferation, migration, and invasion under hypoxia |
Interacts with YBX1 to increase the protein translation of HIF1a under hypoxia |
[125] |
|
VPS9D1-AS1 |
Oncogene |
Facilitates cell proliferation, migration, and stemness |
Sponges miR-491-5p to upregulate SEC61A1 |
[126] |
|
WEE2-AS1 |
Oncogene |
Positively correlated to HBV infection; increases proliferation, migration, invasion, and cell cycle progression |
Upregulates FERMT3 expression and activates PI3K/AKT/GSK3b signaling |
[127] |
|
ZEB1-AS1 |
Oncogene |
Promotes proliferation and invasion, associated with bone metastasis |
Sponges miR-229-3p to upregulate E2F1 expression, sponges miR-23c, sponges miR-302b to increase PI3K-AKT pathway activation and EGFR expression |
|
|
ZEB2-AS1 |
Oncogene |
Associated with large tumor volume, increased tumor-node-metastasis (TNM) stage, and positive lymph node metastasis; promotes proliferation, migration, invasion, and suppressed apoptosis |
Sponges miR-582-5p to upregulate FOXC1 |
[131] |
|
ZFAS1 |
Oncogene |
Associated with worse prognosis and survival; promotes proliferation, migration, and invasion |
Sponges miR-624 to upregulate MDK-mediated ERK/JNK/AKT signaling pathway |
[132] |
|
ZFPM2-AS1 |
Oncogene |
Correlated with advanced TNM stage, distant metastasis, and a poor survival rate; promotes proliferation, migration, invasion, and tumor growth in vivo |
Sponges miR-1226-3p to upregulate ITGB1, sponges miR-3065-5p activity to regulate XRCC4, sponges miR-139 to upregulate GDF10, sponges miR-653 to upregulate GOLM1 |
|
|
ZSCAN16-AS1 |
Oncogene |
Correlated with poor clinical outcomes; promotes proliferation, migration, and invasion |
Sponges miR-451a to increase ATF2 expression; sponges miR-181c-5p to upregulate SPAG9, activating JNK |
|
|
ADORA2A-AS1 |
Tumor suppressor |
Inhibits proliferation, migration, and invasion; represses xenograft growth and metastasis in vivo |
Competitively binds HuR decreasing FSCN1 transcript stability, thereby repressing the AKT pathway |
[139] |
|
CADM1-AS1 |
Tumor suppressor |
Inhibits proliferation, migration, invasion, and tumor growth in vivo |
Regulates the AKT/GSK-3β signaling pathway |
[140] |
|
F11-AS1 |
Tumor suppressor |
Suppresses proliferation, migration, and invasion |
Sponges miR-221-5p to upregulate NR1I3 |
[141] |
|
HHIP-AS1 |
Tumor suppressor |
Downregulation of HHIP-AS1 correlates with larger tumor size, metastasis, and advanced TNM stage; inhibits proliferation, migration, and invasion; induces apoptosis |
Facilitates HHIP mRNA stability by promoting HuR binding to HHIP mRNA |
[45] |
|
HNF1A-AS1 |
Tumor suppressor |
Suppresses proliferation, migration, and invasion; inhibits tumorigenesis and metastasis in vivo |
Interacts and activates SHP-1 |
[142] |
|
MAGI2-AS3 |
Tumor suppressor |
Inhibits proliferation in vitro and tumor growth in vivo |
Sponges miR-519c-3p to increase TXNIP, decreases RCGAP1 expression by facilitating histone demethylation of the RACGAP1 promoter by recruiting KDM1A |
|
|
TMEM220-AS1 |
Tumor suppressor |
Suppresses proliferation and invasion |
Increases TMEM220 expression to regulate Wnt/β-catenin pathway |
[145] |
|
UCHLAS1 |
Tumor suppressor |
Inhibits proliferation and migration |
Enrichment analysis reveals that HRAS, BMP4, and CALM3 are hub genes of HCC, related to UCHLI-AS1 |
[146] |
|
WT1-AS |
Tumor suppressor |
Promotes cell apoptosis |
Inhibits JAK2/STAT3 and MAPK signaling, regulates WT1 by binding promoter region |
[147] |
|
WWOX-AS1 |
Tumor suppressor |
Decreases cell proliferation, migration and EMT |
Sponges miR-20b-5p to upregulate WWOX expression |
[148] |
References
- Dunham, I.; Kundaje, A.; Aldred, S.F.; Collins, P.J.; Davis, C.A.; Doyle, F.; Epstein, C.B.; Frietze, S.; Harrow, J.; Kaul, R.; et al. An Integrated Encyclopedia of DNA Elements in the Human Genome. Nature 2012, 489, 57–74.
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 Catalog of Human Long Noncoding RNAs: Analysis of Their Gene Structure, Evolution, and Expression. Genome Res. 2012, 22, 1775–1789.
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A.; Rinn, J.L. Integrative Annotation of Human Large Intergenic Noncoding RNAs Reveals Global Properties and Specific Subclasses. Genes Dev. 2011, 25, 1915–1927.
- Beermann, J.; Piccoli, M.-T.; Viereck, J.; Thum, T. Non-Coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325.
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297.
- Chang, T.-C.; Mendell, J.T. MicroRNAs in Vertebrate Physiology and Human Disease. Annu. Rev. Genom. Hum. Genet. 2007, 8, 215–239.
- Di Leva, G.; Garofalo, M.; Croce, C.M. MicroRNAs in Cancer. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 287–314.
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates That Thousands of Human Genes Are MicroRNA Targets. Cell 2005, 120, 15–20.
- Alessio, E.; Bonadio, R.S.; Buson, L.; Chemello, F.; Cagnin, S. A Single Cell but Many Different Transcripts: A Journey into the World of Long Non-Coding RNAs. Int. J. Mol. Sci. 2020, 21, 302.
- Volders, P.-J.; Helsens, K.; Wang, X.; Menten, B.; Martens, L.; Gevaert, K.; Vandesompele, J.; Mestdagh, P. LNCipedia: A Database for Annotated Human LncRNA Transcript Sequences and Structures. Nucleic Acids Res. 2013, 41, D246–D251.
- Liu, B.; Xiang, W.; Liu, J.; Tang, J.; Wang, J.; Liu, B.; Long, Z.; Wang, L.; Yin, G.; Liu, J. The Regulatory Role of Antisense LncRNAs in Cancer. Cancer Cell Int. 2021, 21, 459.
- Engström, P.G.; Suzuki, H.; Ninomiya, N.; Akalin, A.; Sessa, L.; Lavorgna, G.; Brozzi, A.; Luzi, L.; Tan, S.L.; Yang, L.; et al. Complex Loci in Human and Mouse Genomes. PLoS Genet. 2006, 2, e47.
- Georg, J.; Hess, W.R. Cis-Antisense RNA, Another Level of Gene Regulation in Bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 286–300.
- Kutter, C.; Watt, S.; Stefflova, K.; Wilson, M.D.; Goncalves, A.; Ponting, C.P.; Odom, D.T.; Marques, A.C. Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression. PLoS Genet. 2012, 8, e1002841.
- Wood, E.; Chin-Inmanu, K.; Jia, H.; Lipovich, L. Sense-Antisense Gene Pairs: Sequence, Transcription, and Structure Are Not Conserved between Human and Mouse. Front. Genet. 2013, 4, 183.
- Yang, Y.; Chen, L.; Gu, J.; Zhang, H.; Yuan, J.; Lian, Q.; Lv, G.; Wang, S.; Wu, Y.; Yang, Y.-C.T.; et al. Recurrently Deregulated LncRNAs in Hepatocellular Carcinoma. Nat. Commun. 2017, 8, 14421.
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global Cancer Statistics. CA Cancer J. Clin. 2011, 61, 69–90.
- Shankaraiah, R.C.; Gramantieri, L.; Fornari, F.; Sabbioni, S.; Callegari, E.; Negrini, M. Animal Models of Hepatocellular Carcinoma Prevention. Cancers 2019, 11, 1792.
- Wong, C.-M.; Tsang, F.H.-C.; Ng, I.O.-L. Non-Coding RNAs in Hepatocellular Carcinoma: Molecular Functions and Pathological Implications. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 137–151.
- Li, D.; Fan, X.; Li, Y.; Yang, J.; Lin, H. The Paradoxical Functions of Long Noncoding RNAs in Hepatocellular Carcinoma: Implications in Therapeutic Opportunities and Precision Medicine. Genes. Dis. 2022, 9, 358–369.
- Cruz, J.A.; Westhof, E. The Dynamic Landscapes of RNA Architecture. Cell 2009, 136, 604–609.
- Novikova, I.V.; Hennelly, S.P.; Tung, C.-S.; Sanbonmatsu, K.Y. Rise of the RNA Machines: Exploring the Structure of Long Non-Coding RNAs. J. Mol. Biol. 2013, 425, 3731–3746.
- Ribeiro, D.M.; Zanzoni, A.; Cipriano, A.; Delli Ponti, R.; Spinelli, L.; Ballarino, M.; Bozzoni, I.; Tartaglia, G.G.; Brun, C. Protein Complex Scaffolding Predicted as a Prevalent Function of Long Non-Coding RNAs. Nucleic Acids Res. 2018, 46, 917–928.
- Morris, K.V. Long Antisense Non-Coding RNAs Function to Direct Epigenetic Complexes That Regulate Transcription in Human Cells. Epigenetics 2009, 4, 296–301.
- Guo, J.-C.; Yang, Y.-J.; Zheng, J.-F.; Zhang, J.-Q.; Guo, M.; Yang, X.; Jiang, X.-L.; Xiang, L.; Li, Y.; Ping, H.; et al. Silencing of Long Noncoding RNA HOXA11-AS Inhibits the Wnt Signaling Pathway via the Upregulation of HOXA11 and Thereby Inhibits the Proliferation, Invasion, and Self-Renewal of Hepatocellular Carcinoma Stem Cells. Exp. Mol. Med. 2019, 51, 1–20.
- Dong, Z.; Li, S.; Wu, X.; Niu, Y.; Liang, X.; Yang, L.; Guo, Y.; Shen, S.; Liang, J.; Guo, W. Aberrant Hypermethylation-Mediated Downregulation of Antisense LncRNA ZNF667-AS1 and Its Sense Gene ZNF667 Correlate with Progression and Prognosis of Esophageal Squamous Cell Carcinoma. Cell Death Dis. 2019, 10, 930.
- Cheng, J.; Wei, D.; Ji, Y.; Chen, L.; Yang, L.; Li, G.; Wu, L.; Hou, T.; Xie, L.; Ding, G.; et al. Integrative Analysis of DNA Methylation and Gene Expression Reveals Hepatocellular Carcinoma-Specific Diagnostic Biomarkers. Genome Med. 2018, 10, 42.
- Mudbhary, R.; Hoshida, Y.; Chernyavskaya, Y.; Jacob, V.; Villanueva, A.; Fiel, M.I.; Chen, X.; Kojima, K.; Thung, S.; Bronson, R.T.; et al. UHRF1 Overexpression Drives DNA Hypomethylation and Hepatocellular Carcinoma. Cancer Cell 2014, 25, 196–209.
- Ye, J.; Fu, Y.; Wang, Z.; Yu, J. Long Non-Coding RNA FOXP4-AS1 Facilitates the Biological Functions of Hepatocellular Carcinoma Cells via Downregulating ZC3H12D by Mediating H3K27me3 through Recruitment of EZH2. Cell Biol. Toxicol. 2022, 38, 1047–1062.
- Gao, J.; Dai, C.; Yu, X.; Yin, X.-B.; Zhou, F. LncRNA LEF1-AS1 Silencing Diminishes EZH2 Expression to Delay Hepatocellular Carcinoma Development by Impairing CEBPB-Interaction with CDCA7. Cell Cycle 2020, 19, 870–883.
- Faghihi, M.A.; Wahlestedt, C. Regulatory Roles of Natural Antisense Transcripts. Nat. Rev. Mol. Cell Biol. 2009, 10, 637–643.
- Guo, Y.; Liu, B.; Huang, T.; Qi, X.; Li, S. HOTAIR Modulates Hepatocellular Carcinoma Progression by Activating FUT8/Core-Fucosylated Hsp90/MUC1/STAT3 Feedback Loop via JAK1/STAT3 Cascade. Dig. Liver Dis. 2023, 55, 113–122.
- Boque-Sastre, R.; Soler, M.; Oliveira-Mateos, C.; Portela, A.; Moutinho, C.; Sayols, S.; Villanueva, A.; Esteller, M.; Guil, S. Head-to-Head Antisense Transcription and R-Loop Formation Promotes Transcriptional Activation. Proc. Natl. Acad. Sci. USA 2015, 112, 5785–5790.
- Li, Y.; Syed, J.; Sugiyama, H. RNA-DNA Triplex Formation by Long Noncoding RNAs. Cell Chem. Biol. 2016, 23, 1325–1333.
- Diederichs, S. The Four Dimensions of Noncoding RNA Conservation. Trends Genet. 2014, 30, 121–123.
- Pan, J.; Wang, R.; Shang, F.; Ma, R.; Rong, Y.; Zhang, Y. Functional Micropeptides Encoded by Long Non-Coding RNAs: A Comprehensive Review. Front. Mol. Biosci. 2022, 9, 817517.
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A CeRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 2011, 146, 353–358.
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The Multilayered Complexity of CeRNA Crosstalk and Competition. Nature 2014, 505, 344–352.
- Chan, J.J.; Tay, Y. Noncoding RNA:RNA Regulatory Networks in Cancer. Int. J. Mol. Sci. 2018, 19, 1310.
- Siniscalchi, C.; Di Palo, A.; Russo, A.; Potenza, N. The LncRNAs at X Chromosome Inactivation Center: Not Just a Matter of Sex Dosage Compensation. Int. J. Mol. Sci. 2022, 23, 611.
- Wong, L.-S.; Wong, C.-M. Decoding the Roles of Long Noncoding RNAs in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 3137.
- Ogawa, Y.; Sun, B.K.; Lee, J.T. Intersection of the RNA Interference and X-Inactivation Pathways. Science 2008, 320, 1336–1341.
- Nolasco, S.; Bellido, J.; Gonçalves, J.; Tavares, A.; Zabala, J.C.; Soares, H. The Expression of Tubulin Cofactor A (TBCA) Is Regulated by a Noncoding Antisense Tbca RNA during Testis Maturation. PLoS ONE 2012, 7, e42536.
- Lipovich, L.; Dachet, F.; Cai, J.; Bagla, S.; Balan, K.; Jia, H.; Loeb, J.A. Activity-Dependent Human Brain Coding/Noncoding Gene Regulatory Networks. Genetics 2012, 192, 1133–1148.
- Bo, C.; Li, X.; He, L.; Zhang, S.; Li, N.; An, Y. A Novel Long Noncoding RNA HHIP-AS1 Suppresses Hepatocellular Carcinoma Progression through Stabilizing HHIP MRNA. Biochem. Biophys. Res. Commun. 2019, 520, 333–340.
- Volders, P.-J.; Anckaert, J.; Verheggen, K.; Nuytens, J.; Martens, L.; Mestdagh, P.; Vandesompele, J. LNCipedia 5: Towards a Reference Set of Human Long Non-Coding RNAs. Nucleic Acids Res. 2019, 47, D135–D139.
- Cai, H.; Zheng, Y.; Wen, Z.; Yang, Y.; Yang, S.; Zhang, Q. LncRNA AIRN Influences the Proliferation and Apoptosis of Hepatocellular Carcinoma Cells by Regulating STAT1 Ubiquitination. Arch. Pharmacol. Res. 2021, 44, 414–426.
- Lu, Q.; Wang, H.; Lei, X.; Ma, Q.; Zhao, J.; Sun, W.; Guo, C.; Huang, D.; Xu, Q. LncRNA ALKBH3-AS1 Enhances ALKBH3 MRNA Stability to Promote Hepatocellular Carcinoma Cell Proliferation and Invasion. J. Cell Mol. Med. 2022, 26, 5292–5302.
- Huang, M.; Chen, W.; Qi, F.; Xia, R.; Sun, M.; Xu, T.; Yin, L.; Zhang, E.; De, W.; Shu, Y. Long Non-Coding RNA ANRIL Is Upregulated in Hepatocellular Carcinoma and Regulates Cell Apoptosis by Epigenetic Silencing of KLF2. J. Hematol. Oncol. 2015, 8, 50.
- Huang, Y.; Xiang, B.; Liu, Y.; Wang, Y.; Kan, H. LncRNA CDKN2B-AS1 Promotes Tumor Growth and Metastasis of Human Hepatocellular Carcinoma by Targeting Let-7c-5p/NAP1L1 Axis. Cancer Lett. 2018, 437, 56–66.
- Huang, D.; Bi, C.; Zhao, Q.; Ding, X.; Bian, C.; Wang, H.; Wang, T.; Liu, H. Knockdown Long Non-Coding RNA ANRIL Inhibits Proliferation, Migration and Invasion of HepG2 Cells by down-Regulation of MiR-191. BMC Cancer 2018, 18, 919.
- Chen, J.; Huang, X.; Wang, W.; Xie, H.; Li, J.; Hu, Z.; Zheng, Z.; Li, H.; Teng, L. LncRNA CDKN2BAS Predicts Poor Prognosis in Patients with Hepatocellular Carcinoma and Promotes Metastasis via the MiR-153-5p/ARHGAP18 Signaling Axis. Aging 2018, 10, 3371–3381.
- Li, K.; Zhao, B.; Wei, D.; Cui, Y.; Qian, L.; Wang, W.; Liu, G. Long Non-Coding RNA ANRIL Enhances Mitochondrial Function of Hepatocellular Carcinoma by Regulating the MiR-199a-5p/ARL2 Axis. Environ. Toxicol. 2020, 35, 313–321.
- Ma, J.; Li, T.; Han, X.; Yuan, H. Knockdown of LncRNA ANRIL Suppresses Cell Proliferation, Metastasis, and Invasion via Regulating MiR-122-5p Expression in Hepatocellular Carcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 205–214.
- Tian, Q.; Yan, X.; Yang, L.; Liu, Z.; Yuan, Z.; Zhang, Y. Long Non-Coding RNA BACE1-AS Plays an Oncogenic Role in Hepatocellular Carcinoma Cells through MiR-214-3p/APLN Axis. Acta Biochim. Biophys. Sin. 2021, 53, 1538–1546.
- Yang, Y.; Ge, H.; Li, D.; Xu, A. E2F1-Induced LncRNA BAIAP2-AS1 Overexpression Contributes to the Malignant Progression of Hepatocellular Carcinoma via MiR-361-3p/SOX4 Axis. Dis. Markers 2021, 2021, e6256369.
- Ma, Y.; Sun, W.; Zhang, Q.; Gao, B.; Cai, W.; Liu, Q.; Liao, J.; Wang, X. LncRNA BSG-AS1 Is Hypoxia-Responsive and Promotes Hepatocellular Carcinoma by Enhancing BSG MRNA Stability. Biochem. Biophys. Res. Commun. 2021, 566, 101–107.
- Feng, Y.; Wei, G.; Zhang, L.; Zhou, H.; Wang, W.; Guo, P.; Cheng, C.; Ji, L.; Cai, Q.; Feng, Y.; et al. LncRNA DARS-AS1 Aggravates the Growth and Metastasis of Hepatocellular Carcinoma via Regulating the MiR-3200-5p-Cytoskeleton Associated Protein 2 (CKAP2) Axis. Bioengineered 2021, 12, 8217–8232.
- Wan, T.; Zheng, J.; Yao, R.; Yang, S.; Zheng, W.; Zhou, P. LncRNA DDX11-AS1 Accelerates Hepatocellular Carcinoma Progression via the MiR-195-5p/MACC1 Pathway. Ann. Hepatol. 2021, 20, 100258.
- Min, J.; Jin, D.; Zhang, F.; Kang, Y.; Qi, Y.; Du, P. DLG1-AS1 Is Activated by MYC and Drives the Proliferation and Migration of Hepatocellular Carcinoma Cells through MiR-497-5p/SSRP1 Axis. Cancer Cell Int. 2021, 21, 16.
- Peng, X.; Wei, F.; Hu, X. Long Noncoding RNA DLGAP1-AS1 Promotes Cell Proliferation in Hepatocellular Carcinoma via Sequestering MiR-486-5p. J. Cell Biochem. 2020, 121, 1953–1962.
- Fu, C.; Li, J.; Li, P.; Cheng, D. LncRNA DNAJC3-AS1 Promotes Hepatocellular Carcinoma (HCC) Progression via Sponging Premature MiR-27b. Cancer Manag. Res. 2021, 13, 8575–8583.
- Liu, X.; Peng, D.; Cao, Y.; Zhu, Y.; Yin, J.; Zhang, G.; Peng, X.; Meng, Y. Upregulated LncRNA DLX6-AS1 Underpins Hepatocellular Carcinoma Progression via the MiR-513c/Cul4A/ANXA10 Axis. Cancer Gene Ther. 2021, 28, 486–501.
- Li, D.; Tang, X.; Li, M.; Zheng, Y. Long Noncoding RNA DLX6-AS1 Promotes Liver Cancer by Increasing the Expression of WEE1 via Targeting MiR-424-5p. J. Cell Biochem. 2019, 120, 12290–12299.
- Ma, Y.-K.; Shen, T.-H.; Yang, X.-Y. Upregulation of LncRNA FAM83H-AS1 in Hepatocellular Carcinoma Promotes Cell Proliferation, Migration and Invasion by Wnt/β-Catenin Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7855–7862.
- Zhuang, J.; He, S.; Wang, G.; Wang, G.; Ni, J.; Zhang, S.; Ye, Y.; Xia, W. Long Noncoding RNA FGFR3-AS1 Promotes Hepatocellular Carcinoma Carcinogenesis via Modulating the PI3K/AKT Pathway. Oncol. Res. 2018, 26, 1257–1265.
- Luo, X.; Zhou, N.; Wang, L.; Zeng, Q.; Tang, H. Long Noncoding RNA GATA3-AS1 Promotes Cell Proliferation and Metastasis in Hepatocellular Carcinoma by Suppression of PTEN, CDKN1A, and TP53. Can. J. Gastroenterol. Hepatol. 2019, 2019, 1389653.
- Zhu, X.-T.; Yuan, J.-H.; Zhu, T.-T.; Li, Y.-Y.; Cheng, X.-Y. Long Noncoding RNA Glypican 3 (GPC3) Antisense Transcript 1 Promotes Hepatocellular Carcinoma Progression via Epigenetically Activating GPC3. FEBS J. 2016, 283, 3739–3754.
- Yang, L.; Zhang, X.; Li, H.; Liu, J. The Long Noncoding RNA HOTAIR Activates Autophagy by Upregulating ATG3 and ATG7 in Hepatocellular Carcinoma. Mol. Biosyst. 2016, 12, 2605–2612.
- Ding, C.; Cheng, S.; Yang, Z.; Lv, Z.; Xiao, H.; Du, C.; Peng, C.; Xie, H.; Zhou, L.; Wu, J.; et al. Long Non-Coding RNA HOTAIR Promotes Cell Migration and Invasion via down-Regulation of RNA Binding Motif Protein 38 in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2014, 15, 4060–4076.
- Gao, J.-Z.; Li, J.; Du, J.-L.; Li, X.-L. Long Non-Coding RNA HOTAIR Is a Marker for Hepatocellular Carcinoma Progression and Tumor Recurrence. Oncol. Lett. 2016, 11, 1791–1798.
- Zhou, J.-J.; Cheng, D.; He, X.-Y.; Meng, Z.; Li, W.-Z.; Chen, R.-F. Knockdown of Hotair Suppresses Proliferation and Cell Cycle Progression in Hepatocellular Carcinoma Cell by Downregulating CCND1 Expression. Mol. Med. Rep. 2017, 16, 4980–4986.
- Wei, S.; Fan, Q.; Yang, L.; Zhang, X.; Ma, Y.; Zong, Z.; Hua, X.; Su, D.; Sun, H.; Li, H.; et al. Promotion of Glycolysis by HOTAIR through GLUT1 Upregulation via MTOR Signaling. Oncol. Rep. 2017, 38, 1902–1908.
- Hu, M.; Fu, Q.; Jing, C.; Zhang, X.; Qin, T.; Pan, Y. LncRNA HOTAIR Knockdown Inhibits Glycolysis by Regulating MiR-130a-3p/HIF1A in Hepatocellular Carcinoma under Hypoxia. Biomed. Pharmacol. 2020, 125, 109703.
- Su, D.-N.; Wu, S.-P.; Chen, H.-T.; He, J.-H. HOTAIR, a Long Non-Coding RNA Driver of Malignancy Whose Expression Is Activated by FOXC1, Negatively Regulates MiRNA-1 in Hepatocellular Carcinoma. Oncol. Lett. 2016, 12, 4061–4067.
- Liu, C.; Shang, Z.; Ma, Y.; Ma, J.; Song, J. HOTAIR/MiR-214-3p/FLOT1 Axis Plays an Essential Role in the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 50–63.
- Zhang, Y.; Xu, J.; Zhang, S.; An, J.; Zhang, J.; Huang, J.; Jin, Y. HOXA-AS2 Promotes Proliferation and Induces Epithelial-Mesenchymal Transition via the MiR-520c-3p/GPC3 Axis in Hepatocellular Carcinoma. Cell Physiol. Biochem. 2018, 50, 2124–2138.
- Ji, W.; Wang, Q.; Yang, J. LncRNA HOXD-AS1 Promotes the Metastasis of Human Hepatocellular Carcinoma via Modulating MiR-326/SLC27A4. Cancer Cell Int. 2020, 20, 161.
- Sun, J.; Guo, Y.; Bie, B.; Zhu, M.; Tian, H.; Tian, J.; Li, J.; Yang, Y.; Ji, F.; Kong, G.; et al. Silencing of Long Noncoding RNA HOXD-AS1 Inhibits Proliferation, Cell Cycle Progression, Migration and Invasion of Hepatocellular Carcinoma Cells through MEK/ERK Pathway. J. Cell Biochem. 2020, 121, 443–457.
- Li, C.; Miao, R.; Zhang, J.; Qu, K.; Liu, C. Long Non-Coding RNA KCNQ1OT1 Mediates the Growth of Hepatocellular Carcinoma by Functioning as a Competing Endogenous RNA of MiR-504. Int. J. Oncol. 2018, 52, 1603–1612.
- Zhang, L.; Wang, L.; Wang, Y.; Chen, T.; Liu, R.; Yang, W.; Liu, Q.; Tu, K. LncRNA KTN1-AS1 Promotes Tumor Growth of Hepatocellular Carcinoma by Targeting MiR-23c/ERBB2IP Axis. Biomed. Pharmacol. 2019, 109, 1140–1147.
- Yin, L.; Chen, Y.; Zhou, Y.; Deng, G.; Han, Y.; Guo, C.; Li, Y.; Zeng, S.; Shen, H. Increased Long Noncoding RNA LASP1-AS Is Critical for Hepatocellular Carcinoma Tumorigenesis via Upregulating LASP1. J. Cell. Physiol. 2019, 234, 13493–13509.
- Dong, H.; Jian, P.; Yu, M.; Wang, L. Silencing of Long Noncoding RNA LEF1-AS1 Prevents the Progression of Hepatocellular Carcinoma via the Crosstalk with MicroRNA-136-5p/WNK1. J. Cell. Physiol. 2020, 235, 6548–6562.
- Feng, Z.; Ye, Z.; Xie, J.; Chen, W.; Li, W.; Xing, C. Study on the Mechanism of LOXL1-AS1/MiR-3614-5p/YY1 Signal Axis in the Malignant Phenotype Regulation of Hepatocellular Carcinoma. Biol. Direct 2021, 16, 24.
- Guo, Y.; Zhong, J.; Wu, F.; Zhan, Z. Long Noncoding RNA MACC1-AS1 Promotes the Stemness of Hepatocellular Carcinoma Cells by Antagonizing MiR-145. J. Int. Med. Res. 2020, 48, 300060520920411.
- Tong, H.; Liu, X.; Li, T.; Qiu, W.; Peng, C.; Shen, B.; Zhu, Z. MACC1-AS1 Promotes Hepatocellular Carcinoma Cell Invasion and Proliferation by Regulating PAX8. Aging 2020, 12, 70–79.
- Ouyang, H.; Zhang, L.; Xie, Z.; Ma, S. Long Noncoding RNA MAFG-AS1 Promotes Proliferation, Migration and Invasion of Hepatocellular Carcinoma Cells through Downregulation of MiR-6852. Exp. Med. 2019, 18, 2547–2553.
- Wang, L.; Sun, L.; Liu, R.; Mo, H.; Niu, Y.; Chen, T.; Wang, Y.; Han, S.; Tu, K.; Liu, Q. Long Non-Coding RNA MAPKAPK5-AS1/PLAGL2/HIF-1α Signaling Loop Promotes Hepatocellular Carcinoma Progression. J. Exp. Clin. Cancer Res. 2021, 40, 72.
- Wang, Y.; Yang, L.; Chen, T.; Liu, X.; Guo, Y.; Zhu, Q.; Tong, X.; Yang, W.; Xu, Q.; Huang, D.; et al. A Novel LncRNA MCM3AP-AS1 Promotes the Growth of Hepatocellular Carcinoma by Targeting MiR-194-5p/FOXA1 Axis. Mol. Cancer 2019, 18, 28.
- Wei, Y.; Wang, Z.; Zong, Y.; Deng, D.; Chen, P.; Lu, J. LncRNA MFI2-AS1 Promotes HCC Progression and Metastasis by Acting as a Competing Endogenous RNA of MiR-134 to Upregulate FOXM1 Expression. Biomed. Pharmacol. 2020, 125, 109890.
- Pan, G.; Zhang, J.; You, F.; Cui, T.; Luo, P.; Wang, S.; Li, X.; Yuan, Q. ETS Proto-Oncogene 1-Activated Muskelin 1 Antisense RNA Drives the Malignant Progression of Hepatocellular Carcinoma by Targeting MiR-22-3p to Upregulate ETS Proto-Oncogene 1. Bioengineered 2022, 13, 1346–1358.
- Gao, W.; Chen, X.; Chi, W.; Xue, M. Long Non-coding RNA MKLN1-AS Aggravates Hepatocellular Carcinoma Progression by Functioning as a Molecular Sponge for MiR-654-3p, Thereby Promoting Hepatoma-derived Growth Factor Expression. Int. J. Mol. Med. 2020, 46, 1743–1754.
- Teng, F.; Zhang, J.-X.; Chang, Q.-M.; Wu, X.-B.; Tang, W.-G.; Wang, J.-F.; Feng, J.-F.; Zhang, Z.-P.; Hu, Z.-Q. LncRNA MYLK-AS1 Facilitates Tumor Progression and Angiogenesis by Targeting MiR-424-5p/E2F7 Axis and Activating VEGFR-2 Signaling Pathway in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 235.
- Liu, J.; Zhao, S.-Y.; Jiang, Q.; Qu, Y.; Huang, X.; Du, J.; Sun, W.; Ye, Q. Long Noncoding RNA MYLK-AS1 Promotes Growth and Invasion of Hepatocellular Carcinoma through the EGFR/HER2-ERK1/2 Signaling Pathway. Int. J. Biol. Sci. 2020, 16, 1989–2000.
- Wang, Y.; Yang, L.; Dong, X.; Yang, X.; Zhang, X.; Liu, Z.; Zhao, X.; Wen, T. Overexpression of NNT-AS1 Activates TGF-β Signaling to Decrease Tumor CD4 Lymphocyte Infiltration in Hepatocellular Carcinoma. Biomed. Res. Int. 2020, 2020, 8216541.
- Lu, Y.-B.; Jiang, Q.; Yang, M.-Y.; Zhou, J.-X.; Zhang, Q. Long Noncoding RNA NNT-AS1 Promotes Hepatocellular Carcinoma Progression and Metastasis through MiR-363/CDK6 Axis. Oncotarget 2017, 8, 88804–88814.
- He, H.; Chen, T.; Mo, H.; Chen, S.; Liu, Q.; Guo, C. Hypoxia-Inducible Long Noncoding RNA NPSR1-AS1 Promotes the Proliferation and Glycolysis of Hepatocellular Carcinoma Cells by Regulating the MAPK/ERK Pathway. Biochem. Biophys. Res. Commun. 2020, 533, 886–892.
- Li, X.; Li, Y.; Bai, S.; Zhang, J.; Liu, Z.; Yang, J. NR2F1-AS1/MiR-140/HK2 Axis Regulates Hypoxia-Induced Glycolysis and Migration in Hepatocellular Carcinoma. Cancer Manag. Res. 2021, 13, 427–437.
- Kong, S.; Xue, H.; Li, Y.; Li, P.; Ma, F.; Liu, M.; Li, W. The Long Noncoding RNA OTUD6B-AS1 Enhances Cell Proliferation and the Invasion of Hepatocellular Carcinoma Cells through Modulating GSKIP/Wnt/β-Catenin Signalling via the Sequestration of MiR-664b-3p. Exp. Cell Res. 2020, 395, 112180.
- Yuan, S.-X.; Tao, Q.-F.; Wang, J.; Yang, F.; Liu, L.; Wang, L.-L.; Zhang, J.; Yang, Y.; Liu, H.; Wang, F.; et al. Antisense Long Non-Coding RNA PCNA-AS1 Promotes Tumor Growth by Regulating Proliferating Cell Nuclear Antigen in Hepatocellular Carcinoma. Cancer Lett. 2014, 349, 87–94.
- Sun, J.; Zhang, Y.; Li, B.; Dong, Y.; Sun, C.; Zhang, F.; Jin, L.; Chen, D.; Wang, W. PITPNA-AS1 Abrogates the Inhibition of MiR-876-5p on WNT5A to Facilitate Hepatocellular Carcinoma Progression. Cell Death Dis. 2019, 10, 844.
- Ou, Y.; Deng, Y.; Wang, H.; Zhang, Q.; Luo, H.; Hu, P. Targeting Antisense LncRNA PRKAG2-AS1, as a Therapeutic Target, Suppresses Malignant Behaviors of Hepatocellular Carcinoma Cells. Front. Med. 2021, 8, 649279.
- Qin, M.; Meng, Y.; Luo, C.; He, S.; Qin, F.; Yin, Y.; Huang, J.; Zhao, H.; Hu, J.; Deng, Z.; et al. LncRNA PRR34-AS1 Promotes HCC Development via Modulating Wnt/β-Catenin Pathway by Absorbing MiR-296-5p and Upregulating E2F2 and SOX12. Mol. Ther.-Nucleic Acids 2021, 25, 37–52.
- Zhang, Z.; Zhou, Y.; Jia, Y.; Wang, C.; Zhang, M.; Xu, Z. PRR34-AS1 Promotes Exosome Secretion of VEGF and TGF-β via Recruiting DDX3X to Stabilize Rab27a MRNA in Hepatocellular Carcinoma. J. Transl. Med. 2022, 20, 491.
- Yang, X.; Song, D.; Zhang, J.; Yang, X.; Feng, H.; Guo, J. PRR34-AS1 Sponges MiR-498 to Facilitate TOMM20 and ITGA6 Mediated Tumor Progression in HCC. Exp. Mol. Pathol. 2021, 120, 104620.
- Mu, J.-Y.; Tian, X.-J.; Chen, Y.-J. LncRNA RBM5-AS1 Promotes Cell Proliferation and Invasion by Epigenetically Silencing MiR-132/212 in Hepatocellular Carcinoma Cells. Cell Biol. Int. 2021, 45, 2201–2210.
- Zhang, X.; Yan, Z.; Wang, L.; Zhang, S.; Gao, M. STAT1-Induced Upregulation of LncRNA RHPN1-AS1 Predicts a Poor Prognosis of Hepatocellular Carcinoma and Contributes to Tumor Progression via the MiR-485/CDCA5 Axis. J. Cell Biochem. 2020, 121, 4741–4755.
- Fen, H.; Hongmin, Z.; Wei, W.; Chao, Y.; Yang, Y.; Bei, L.; Zhihua, S. RHPN1-AS1 Drives the Progression of Hepatocellular Carcinoma via Regulating MiR-596/IGF2BP2 Axis. Curr. Pharmacol. Des. 2020, 25, 4630–4640.
- Huang, C.; Li, K.; Huang, R.; Zhu, J.; Yang, J. RNF185-AS1 Promotes Hepatocellular Carcinoma Progression through Targeting MiR-221-5p/Integrin Β5 Axis. Life Sci. 2021, 267, 118928.
- Li, Y.; Liu, G.; Li, X.; Dong, H.; Xiao, W.; Lu, S. Long Non-Coding RNA SBF2-AS1 Promotes Hepatocellular Carcinoma Progression through Regulation of MiR-140-5p-TGFBR1 Pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2826–2832.
- Li, Y.; Guo, D.; Lu, G.; Mohiuddin Chowdhury, A.T.M.; Zhang, D.; Ren, M.; Chen, Y.; Wang, R.; He, S. LncRNA SNAI3-AS1 Promotes PEG10-Mediated Proliferation and Metastasis via Decoying of MiR-27a-3p and MiR-34a-5p in Hepatocellular Carcinoma. Cell Death Dis. 2020, 11, 685.
- Zhang, W.; Wu, Y.; Hou, B.; Wang, Y.; Deng, D.; Fu, Z.; Xu, Z. A SOX9-AS1/MiR-5590-3p/SOX9 Positive Feedback Loop Drives Tumor Growth and Metastasis in Hepatocellular Carcinoma through the Wnt/β-Catenin Pathway. Mol. Oncol. 2019, 13, 2194–2210.
- Di Palo, A.; Siniscalchi, C.; Mosca, N.; Russo, A.; Potenza, N. A Novel CeRNA Regulatory Network Involving the Long Non-Coding Antisense RNA SPACA6P-AS, MiR-125a and Its MRNA Targets in Hepatocarcinoma Cells. Int. J. Mol. Sci. 2020, 21, 5068.
- Kuai, J.; Zheng, L.; Yi, X.; Liu, Z.; Qiu, B.; Lu, Z.; Jiang, Y. ST8SIA6-AS1 Promotes the Development of Hepatocellular Carcinoma Cells through MiR-338-3p/NONO Axis. Dig. Liver Dis. 2021, 53, 1192–1200.
- Li, Y.; Jiang, A. ST8SIA6-AS1 Promotes Hepatocellular Carcinoma by Absorbing MiR-5195-3p to Regulate HOXB6. Cancer Biol. Ther. 2020, 21, 647–655.
- Huang, W.; Chen, Q.; Dai, J.; Zhang, Y.; Yi, Y.; Wei, X. Long Noncoding TMPO Antisense RNA 1 Promotes Hepatocellular Carcinoma Proliferation and Epithelial-Mesenchymal Transition by Targeting the MicroRNA-126-3p/LRP6/β-Catenin Axis. Ann. Transl. Med. 2021, 9, 1679.
- Guo, X.; Wang, Y. LncRNA TMPO-AS1 Promotes Hepatocellular Carcinoma Cell Proliferation, Migration and Invasion through Sponging MiR-329-3p to Stimulate FOXK1-Mediated AKT/MTOR Signaling Pathway. Cancer Med. 2020, 9, 5235–5246.
- Wang, Z.; Huang, D.; Huang, J.; Nie, K.; Li, X.; Yang, X. LncRNA TMPO-AS1 Exerts Oncogenic Roles in HCC Through Regulating MiR-320a/SERBP1 Axis. OncoTargets Ther. 2020, 13, 6539–6551.
- Li, S.; Huang, Y.; Huang, Y.; Fu, Y.; Tang, D.; Kang, R.; Zhou, R.; Fan, X.-G. The Long Non-Coding RNA TP73-AS1 Modulates HCC Cell Proliferation through MiR-200a-Dependent HMGB1/RAGE Regulation. J. Exp. Clin. Cancer Res. 2017, 36, 51.
- Sun, X.; Qian, Y.; Wang, X.; Cao, R.; Zhang, J.; Chen, W.; Fang, M. LncRNA TRG-AS1 Stimulates Hepatocellular Carcinoma Progression by Sponging MiR-4500 to Modulate BACH1. Cancer Cell Int. 2020, 20, 367.
- Zhou, C.; Chen, Z.; Peng, C.; Chen, C.; Li, H. Long Noncoding RNA TRIM52-AS1 Sponges MiR-514a-5p to Facilitate Hepatocellular Carcinoma Progression Through Increasing MRPS18A. Cancer Biother. Radiopharm. 2021, 36, 211–219.
- Zhu, X.; Jiang, S.; Wu, Z.; Liu, T.; Zhang, W.; Wu, L.; Xu, L.; Shao, M. Long Non-Coding RNA TTN Antisense RNA 1 Facilitates Hepatocellular Carcinoma Progression via Regulating MiR-139-5p/SPOCK1 Axis. Bioengineered 2021, 12, 578–588.
- Huang, X.; Pan, J.; Wang, G.; Huang, T.; Li, C.; Wang, Y.; Li, X. UNC5B-AS1 Promotes the Proliferation, Migration and EMT of Hepatocellular Carcinoma Cells via Regulating MiR-4306/KDM2A Axis. Cell Cycle 2021, 20, 2114–2124.
- Zhang, D.-Y.; Sun, Q.-C.; Zou, X.-J.; Song, Y.; Li, W.-W.; Guo, Z.-Q.; Liu, S.-S.; Liu, L.; Wu, D.-H. Long Noncoding RNA UPK1A-AS1 Indicates Poor Prognosis of Hepatocellular Carcinoma and Promotes Cell Proliferation through Interaction with EZH2. J. Exp. Clin. Cancer Res. 2020, 39, 229.
- Chen, S.-P.; Zhu, G.-Q.; Xing, X.-X.; Wan, J.-L.; Cai, J.-L.; Du, J.-X.; Song, L.-N.; Dai, Z.; Zhou, J. LncRNA USP2-AS1 Promotes Hepatocellular Carcinoma Growth by Enhancing YBX1-Mediated HIF1α Protein Translation Under Hypoxia. Front. Oncol. 2022, 12, 882372.
- Fa, X.; Song, P.; Fu, Y.; Deng, Y.; Liu, K. Long Non-Coding RNA VPS9D1-AS1 Facilitates Cell Proliferation, Migration and Stemness in Hepatocellular Carcinoma. Cancer Cell Int. 2021, 21, 131.
- Hu, Z.; Huang, P.; Yan, Y.; Zhou, Z.; Wang, J.; Wu, G. Hepatitis B Virus X Protein Related LncRNA WEE2-AS1 Promotes Hepatocellular Carcinoma Proliferation and Invasion. Biochem. Biophys. Res. Commun. 2019, 508, 79–86.
- Mu, B.; Lv, C.; Liu, Q.; Yang, H. Long Non-Coding RNA ZEB1-AS1 Promotes Proliferation and Metastasis of Hepatocellular Carcinoma Cells by Targeting MiR-299-3p/E2F1 Axis. J. Biochem. 2021, 170, 41–50.
- Xue, S.; Lu, F.; Sun, C.; Zhao, J.; Zhen, H.; Li, X. LncRNA ZEB1-AS1 Regulates Hepatocellular Carcinoma Progression by Targeting MiR-23c. World J. Surg. Oncol. 2021, 19, 121.
- Ma, Z.-J.; Wang, Y.; Li, H.-F.; Liu, M.-H.; Bi, F.-R.; Ma, L.; Ma, H.; Yan, H.-L. LncZEB1-AS1 Regulates Hepatocellular Carcinoma Bone Metastasis via Regulation of the MiR-302b-EGFR-PI3K-AKT Axis. J. Cancer 2020, 11, 5118–5128.
- Wu, S.M.; Chen, J.; Liang, Y.; Luo, Q.; Tong, Y.Y.; Xie, L. Long Non-Coding RNA ZEB2-AS1 Promotes Hepatocellular Carcinoma Progression by Regulating The MiR-582-5p/FOXC1 Axis. Cell J. 2022, 24, 285–293.
- Duan, R.; Li, C.; Wang, F.; Han, F.; Zhu, L. The Long Noncoding RNA ZFAS1 Potentiates the Development of Hepatocellular Carcinoma via the MicroRNA-624/MDK/ERK/JNK/P38 Signaling Pathway. Onco Targets 2020, 13, 4431–4444.
- Liu, W.; Zhang, G.-Q.; Zhu, D.-Y.; Wang, L.-J.; Li, G.-T.; Xu, J.-G.; Jin, X.-L.; Zhu, Y.-M.; Yang, X.-Y. Long Noncoding RNA ZFPM2-AS1 Regulates ITGB1 by MiR-1226-3p to Promote Cell Proliferation and Invasion in Hepatocellular Carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7612–7620.
- Liu, J.; Zhang, H.; Xia, P.; Zhu, Y.; Xu, K.; Liu, Z.; Yuan, Y. Genome Stability-related LncRNA ZFPM2-AS1 Promotes Tumor Progression via MiR-3065-5p/XRCC4 in Hepatocellular Carcinoma. Int. J. Oncol. 2023, 62, 19.
- He, H.; Wang, Y.; Ye, P.; Yi, D.; Cheng, Y.; Tang, H.; Zhu, Z.; Wang, X.; Jin, S. Long Noncoding RNA ZFPM2-AS1 Acts as a MiRNA Sponge and Promotes Cell Invasion through Regulation of MiR-139/GDF10 in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 159.
- Zhang, X.-W.; Li, Q.-H.; Xu, Z.; Dou, J.-J. STAT1-Induced Regulation of LncRNA ZFPM2-AS1 Predicts Poor Prognosis and Contributes to Hepatocellular Carcinoma Progression via the MiR-653/GOLM1 Axis. Cell Death Dis. 2021, 12, 31.
- Lv, C.; Wan, Q.; Shen, C.; Wu, H.; Zhou, B.; Wang, W. Long Non-coding RNA ZSCAN16-AS1 Promotes the Malignant Properties of Hepatocellular Carcinoma by Decoying MicroRNA-451a and Consequently Increasing ATF2 Expression. Mol. Med. Rep. 2021, 24, 780.
- Liu, J.; Liu, R.; Liu, Y.; Li, L.; Cao, H.; Liu, J.; Cao, G. ZSCAN16-AS1 Expedites Hepatocellular Carcinoma Progression via Modulating the MiR-181c-5p/SPAG9 Axis to Activate the JNK Pathway. Cell Cycle 2021, 20, 1134–1146.
- Pu, J.; Zhang, Y.; Wang, A.; Qin, Z.; Zhuo, C.; Li, W.; Xu, Z.; Tang, Q.; Wang, J.; Wei, H. ADORA2A-AS1 Restricts Hepatocellular Carcinoma Progression via Binding HuR and Repressing FSCN1/AKT Axis. Front. Oncol. 2021, 11, 4081.
- Wang, F.; Qi, X.; Li, Z.; Jin, S.; Xie, Y.; Zhong, H. LncRNA CADM1-AS1 Inhibits Cell-Cycle Progression and Invasion via PTEN/AKT/GSK-3β Axis in Hepatocellular Carcinoma. Cancer Manag. Res. 2019, 11, 3813–3828.
- Deng, Y.; Wei, Z.; Huang, M.; Xu, G.; Wei, W.; Peng, B.; Nong, S.; Qin, H. Long Non-Coding RNA F11-AS1 Inhibits HBV-Related Hepatocellular Carcinoma Progression by Regulating NR1I3 via Binding to MicroRNA-211-5p. J. Cell Mol. Med. 2020, 24, 1848–1865.
- Ding, C.-H.; Yin, C.; Chen, S.-J.; Wen, L.-Z.; Ding, K.; Lei, S.-J.; Liu, J.-P.; Wang, J.; Chen, K.-X.; Jiang, H.-L.; et al. The HNF1α-Regulated LncRNA HNF1A-AS1 Reverses the Malignancy of Hepatocellular Carcinoma by Enhancing the Phosphatase Activity of SHP-1. Mol. Cancer 2018, 17, 63.
- Wei, H.; Tang, Q.; Wang, A.; Zhang, Y.; Qin, Z.; Li, W.; Xu, Z.; Wang, J.; Pu, J. LncRNA MAGI2-AS3 Exerts Antioncogenic Roles in Hepatocellular Carcinoma via Regulating the MiR-519c-3p/TXNIP Axis. J. Oncol. 2021, 2021, 5547345.
- Pu, J.; Wang, J.; Wei, H.; Lu, T.; Wu, X.; Wu, Y.; Shao, Z.; Luo, C.; Lu, Y. LncRNA MAGI2-AS3 Prevents the Development of HCC via Recruiting KDM1A and Promoting H3K4me2 Demethylation of the RACGAP1 Promoter. Mol. Ther.-Nucleic Acids 2019, 18, 351–362.
- Liu, Y.; Liu, R.; Zhao, J.; Zeng, Z.; Shi, Z.; Lu, Q.; Guo, J.; Li, L.; Yao, Y.; Liu, X.; et al. LncRNA TMEM220-AS1 Suppresses Hepatocellular Carcinoma Cell Proliferation and Invasion by Regulating the TMEM220/β-Catenin Axis. J. Cancer 2021, 12, 6805–6813.
- Zhang, R.; Wei, Y.; Zhu, L.; Huang, L.; Wei, Y.; Chen, G.; Dang, Y.; Feng, Z. LncRNA UCHL1-AS1 Prevents Cell Mobility of Hepatocellular Carcinoma: A Study Based on in Vitro and Bioinformatics. Int. J. Clin. Exp. Pathol. 2018, 11, 2270–2280.
- Lv, L.; Chen, G.; Zhou, J.; Li, J.; Gong, J. WT1-AS Promotes Cell Apoptosis in Hepatocellular Carcinoma through down-Regulating of WT1. J. Exp. Clin. Cancer Res. 2015, 34, 119.
- Xu, D.; Liu, X.; Wu, J.; Wang, Y.; Zhou, K.; Chen, W.; Chen, J.; Chen, C.; Chen, L. LncRNA WWOX-AS1 Sponges MiR-20b-5p in Hepatocellular Carcinoma and Represses Its Progression by Upregulating WWOX. Cancer Biol. Ther. 2020, 21, 927–936.
