Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Serafeim - Chrysovalantis Kotoulas.
Fungal diseases range from relatively minor superficial and mucosal infections to severe, life-threatening systemic infections. Delayed diagnosis and treatment could result in serious consequences for patient outcomes and could be associated with high medical costs. Invasive pulmonary aspergillosis (IPA) is a frequent complication of critically ill patients with H1N1 virus infection and severe respiratory failure. Invasive pulmonary aspergillosis (IPA) presents a known risk to critically ill patients with SARS-CoV-2.
- invasive pulmonary aspergillosis
- COVID-19
- SARS-CoV-2
- treatment options
1. Diagnostic Criteria
Early initiation of appropriate antifungal treatment remains a major predictor of outcomes in invasive fungal infections (IFIs) and is pivotal for successful treatment; however, many uncertainties exist regarding the identification and diagnosis of CAPAOVID-19-associated pulmonary aspergillosis (CAPA) [28][1]. Early diagnosis of IFIs is still difficult, despite novel breakthroughs in diagnostic procedures, especially prior to the development of a typical radiological image. There is also an extreme difficulty in the differential diagnosis between the colonization by Aspergillus and invasive IPApulmonary aspergillosis (IPA), especially in ICU intensive care unit (ICU) patients. Thus, due to the absence of a ‘‘gold standard’’, the diagnosis of IPA remains a strenuous challenge, as it depends on clinical and microbiological data, along with histopathology when feasible [27][2].
Ideally, screening for CAPA includes the use of a combination of imaging methods (X-ray, CT scan) with Aspergillus antigen tests in bronchoalveolar lavage (BAL) and serum, including galactomannan (GM), lateral flow tests, or Aspergillus polymerase chain PCRreaction (PCR) tests [35,49,50,51][3][4][5][6]. However, the use of imaging and of other diagnostic methods must be balanced with the risks for other patients and healthcare workers during the process of obtaining samples, as well as for the patients themselves, during their transport and stay inside the CT scan room. Pronounced hypoxemia frequently prohibits the transferring of patients for diagnostic CT scans; BAL sampling also poses risks because of possible virus dispersion. Additional issues, which could complicate the diagnostic approach in COVID-19 disease, include a shortage of standard equipment for microbiological examinations and a lack of expert professionals to precisely identify the specific fungal infections [27,52][2][7].
Recently, a panel, including 29 international experts, reviewed current insights into the diagnosis and management of IAPA in ICU patients and proposed a case definition of IAPA, which would be appropriate to use in clinical studies, focusing on four main areas: (a) entry criteria, (b) host factors, (c) clinical features, and (d) mycological evidence of infection [27][2]. Firstly, in addition to a positive diagnostic test for influenza, patients would require having a clinical symptomatology compatible with influenza disease and respiratory distress syndrome during a timescale between one week before ICU admission and 72–96 h post-admission. Secondly, host factors referred to the EORTC/MSGERC definition and AspICU algorithm [47[8][9],53], were not considered as a key element of the diagnostic process and have not been included in the consensus definition for IAPA, despite the fact that most IAPA cases have at least one underlying condition, such as steroid use, diabetes mellitus or obesity. Thirdly, the authors pointed out, that the distinction between proven and probable IAPA is of utmost importance for clinical trials, while in clinical practice, clinicians should not distinguish between proven and probable disease. The authors reported tracheobronchitis as a separate entity, characterized by tracheal or bronchial ulcerations or nodules, the presence of hyphal elements suggestive of Aspergillus on pseudomembranes, or the presence of plaques, visualized during bronchoscopy. The proposed criteria for the proven disease include the fulfillment of the entry criterion, combined with histological evidence of invasive fungal elements, in biopsy or in brush specimens (airway plaques, pseudomembranes, or ulcers with hyphal elements) and mycological evidence for the presence of Aspergillus (Aspergillus growth on culture, or positive Aspergillus PCR in tissue). In patients with pulmonary infiltrates or endobronchial plaques, the diagnosis of probable IAPA should be confirmed by a positive GM test, obtained from a BAL sample, or positive culture of a sample from a tracheal aspirate. A serum GM index cutoff >0.5 and a BAL GM index cutoff ≥1.0 are recommended cutoff values that ensure high specificity and acceptable sensitivity, a fact that is also consistent with other recommendations [47,50][5][8]. A positive culture of an upper airway sample is considered indicative of the diagnosis of probable IAPA. However, the diagnosis should be confirmed with serum, BAL GM test, or positive BAL culture to mitigate the risk of overdiagnosis and over-treatment. In patients with tracheobronchitis, the presence of pulmonary infiltrates on chest X-ray, or other imaging methods, is not required to raise suspicion of probable disease. The basic steps of the diagnostic process of CAPA are presented in Figure 1.
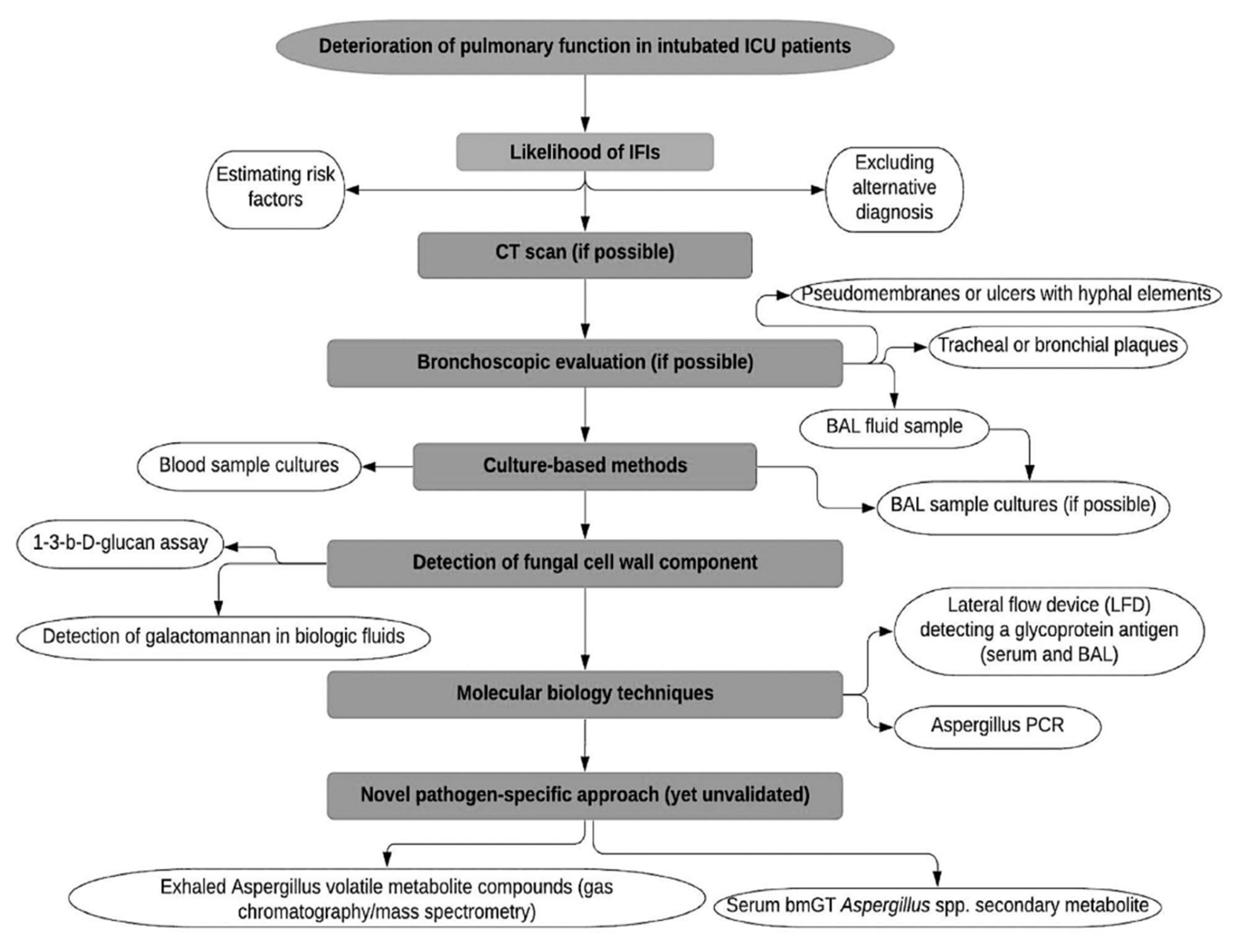
Figure 1. Diagnostic process of COVID-19-associated pulmonary aspergillosis. BAL: Bronchoalveolar lavage, ICU: Intensive Care Unit, IFIs: Invasive Fungal Infections, CT: Computed Tomography, LFD: Lateral Flow Device, PCR, Polymerase Chain Reaction.
Several fungal pathogens that cause invasive infections present similar morphology to Aspergillus, making its histopathological identification challenging. As a result, only the culture growth of the pathogen in question can definitively confirm the cause of the infection [54][10]. To make things worse, biopsy samples, which are necessary to achieve a diagnosis based on culture growth, are not easily obtainable in patients with SARS-CoV-2 and even when they are available, they do not always provide living microorganisms suitable for culture growth [36][11].
EORTC criteria for probable IFI include direct mycological tests, such as direct microscopy, culture or cytology, and indirect mycological tests, such as cell wall constituents, or antigen detection, as well as detection of β-D-glucan in serum, or of GM in serum, plasma, cerebrospinal fluid (CSF), or BAL [55][12].
Conventional microscopic examination and qualitative culture of respiratory tract samples have quite low sensitivity and specificity (around 50%) [39][13]. Additionally, respiratory tract cultures, even when obtained by BAL, may reflect airway colonization, and require a prolonged period of incubation, before yielding diagnostic data [56][14].
The spread of Aspergillus through vessels is a key characteristic of its pathogenesis, which allows the immunological tracking of the fungi via the detection of specific antigens in BAL or serum, namely, the galactomannan enzyme immunoassay (GM-EIA), and a ‘‘pan-fungal’’ assay, which detect Aspergillus GM and (1 → 3)-β-D-glucan, a preserved component of the fungal cell wall, respectively [54,57,58][10][15][16]. A prospective single-center study by Meersseman et al. investigated the role of GM in BAL fluid and serum, as a tool for early diagnosis of IA in the ICU; by using a cut-off index of 0.5, the sensitivity and specificity of GM detection in BAL fluid was 88% and 87%, respectively. In comparison, the sensitivity of serum GM was only 42% [59][17]. In 11 out of 26 proven IA cases, BAL culture and serum GM remained negative, whereas GM in BAL was positive. The authors concluded that GM detection in BAL fluid seems to be useful in establishing the diagnosis of IA in the ICU settings. In the retrospective multicenter cohort study by Schauwvlieghe et al. [14][18] that included adult patients with severe influenza admitted to seven ICUs across Belgium and the Netherlands, serum GM testing performed better with 20/31 positive cases (65%), nevertheless, BAL GM remained superior with 67/76 positive cases (88%). Rutsaert et al. [34][19], in a small study on CAPA, acquired bronchial aspirates or bronchoscopy-guided biopsies of suspicious lesions while performing bronchoscopic procedures due to various causes, such as respiratory deterioration or atelectasis. Subsequently, GM assays on BAL and serum were routinely assessed. IPA was diagnosed via histopathology in four patients all of whom presented positive GM in BAL but negative in serum (<0.5), concluding that the BAL GM test is probably superior to that of serum in the diagnosis of CAPA. Koehler and colleagues described IA in five out of nineteen patients admitted to their ICU (26%); three patients were identified as positive for Aspergillus spp. with PCR and GM from a BAL sample, one patient grew Aspergillus spp. on a tracheal aspirate, but was negative for serum GM and the final patient had positive serum GM with no growth on a tracheal aspirate [19][20]. Alanio and colleagues described nine out of twenty-seven SARS-CoV-2 patients (33%), admitted to their ICU, as having IA [17][21]. However, only one patient, with concurrent candidemia (C. glabrata), received antifungal treatment with voriconazole. Supportive diagnostic criteria, including serum GM and BAL GM, were negative in all patients and no deaths were attributed to IFI.
It has been reported that serum GM detection for the diagnosis of IA in COVID-19 patients is less sensitive than in influenza patients and GM testing is not sufficiently validated for upper respiratory tract samples [60][22]. A positive serum GM result (≥0.5) would be highly suspicious for CAPA, although a negative one should not be used to exclude the diagnosis [35,59][3][17].
Next-generation monoclonal antibody (MAb)-based assays were recently developed due to the problematic accuracy of the indirect tests. By using hybridoma technology, these assays detect Mab specific for Aspergillus. They have been used in the development of an immuno-chromatographic lateral flow device (LFD) for the diagnosis of IPA in the point-of-care (POC) [61][23]. The LFD test specific for Aspergillus is based on the JF5 Ab and detects an antigen that is a glycoprotein secreted extracellularly during active growth of Aspergillus spp. Since MAb binds to an extracellular substance which is secreted solely during fungus multiplication, this test provides the advantage of detecting only active strains. The LFD presented increased sensitivity and specificity compared to the β-D-glucan and GM assays, proving its usefulness in the diagnosis of IPA in various studies [54,61][10][23]. In addition to that, a similar monoclonal Ab476-based LFD for urine antigen detection has also been manufactured, although it requires additional validation [62,63][24][25].
Recent recommendations of the American Thoracic Society Assembly on pulmonary infections and tuberculosis stated that in immunocompromised adult patients who are suspected of having IPA the use of blood or serum Aspergillus PCR testing is recommended (strong recommendation, high-quality evidence) [50][5]. In patients with severe immunocompromising conditions, the recommendations suggest the inclusion of Aspergillus PCR in BAL testing as part of the evaluation (strong recommendation, high-quality evidence).
There is no clear evidence on how the empirical use of antifungal therapy in critically ill patients impacts PCR test performance since PCR can detect very low copy numbers. While the use of antifungal drugs seems to reduce the sensitivity of GM testing for IPA, the ability of PCR to detect low copy numbers makes it, possibly, an attractive option for assessing patients who receive active antifungal therapy. However, the high sensitivity of BAL-PCR makes it difficult to discriminate between IPA and simple Aspergillus colonization [50][5]. Furthermore, during bronchoscopy, an aerosol is developed, making it a hazardous procedure for viral contamination in COVID-19 units. As a result, it has been suggested that it should only be used when a definite diagnosis is required to change clinical management and samples obtained from the upper respiratory tract are negative [64][26]. In such cases, the ratio between the risk of viral transmission and the benefit of achieving the optimal diagnosis should be balanced in order to attain the best possible patient care.
Novel diagnostic biochemical markers, based on the detection of metabolites of Aspergillus spp. had recently been introduced. Filamentous fungi, including Aspergillus species, can produce an array of secondary metabolites, many of which are volatile [65][27]. These volatile organic compounds (VOCs) could identify evidence of Aspergillus metabolism in the breath of patients with IA [36,66,67][11][28][29]. Gliotoxin (GT), a secondary metabolite of Aspergillus fumigatus, and bis(methylthio)gliotoxin (bmGT), a degradation product of gliotoxin, have been proposed as potential biomarkers for IPA diagnosis [36,66][11][28]. However, recently published data showed a very poor performance of these biomarkers for diagnosing IPA [68][30], a fact that is not supportive of the use of serum or BAL GT/bmGT in routine practice.
Varying diagnostic performance, availability, and time-to-results turnaround time are important limitations of currently approved biomarkers and molecular assays for the diagnosis of IA. Specific characteristics of different diagnostic tests for CAPA are presented in Table 1.
Table 1. Diagnostic tests for CAPA, features and pitfalls [36,49,50,51,55,56,63,66,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87].
Diagnostic tests for CAPA, features and pitfalls [4][5][6][11][12][14][25][28][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49].
| Tests | Features | Diagnostic Value | Turnaround | Pitfalls |
|---|---|---|---|---|
| Conventional microscopic examination [36,49,50,51,69,70][4][5][6][11][31][32] |
Availability. Simplicity. Low cost. |
Suboptimal, low to moderate sensitivity and predictive value. | Rapid. | Challenging to differentiate between infection and colonization. May reflect airway colonization. |
| Respiratory sample cultures [49,50,][4][51,5][56,6][14]70[32] |
Simplicity. Low cost. Identification of species. Antifungal susceptibility testing. |
Suboptimal, low to moderate sensitivity and predictive value. | Prolonged. | Challenging to differentiate between infection and colonization. |
| Galactomannan (GM) in biologic fluids [36,49,51,55,469,71,][672,][1173,]74,75][[12][31][33][34][35][36][37] | Serum: Low or moderate sensitivity depending on the index cut-off used. Moderate specificity. Better performance in neutropenic than in non-neutropenic patients. BAL: Moderate or high sensitivity and high specificity of 81–96.6% depending on the optical density index cut-off used, sensitivity exceeds 70% in most studies. Raising the cutoff improves test specificity without compromising sensitivity. High NPV, moderate or high PPV. |
Variable. | Variable performance. BAL: Optimal threshold has not been determined; sensitivity may be reduced in the presence of antifungals. |
|
| Serum 1-3-b-D-glucan (BDG) assay [36,49,51,66,76][4][6][11][28][38] |
Low or moderate sensitivity (49.6–80%), good specificity (82–98.9%), acceptable PPV (83.5%), high NPV (89–94.6%) (useful to exclude diagnosis rather than confirm it). | Variable. | False-positive results (b-lactam antibiotics, human blood products, immunoglobulin, albumin plasma, cellulose hemodialysis membranes, bacterial bloodstream infections, e.g., Pseudomonas aeruginosa) |
|
| PCR-based methods [36,49,51,70,[11]77,[32]78,[39]79,[40]80,81,82][4][6][41][42][43][44] |
High cost. Not affected by the immune status of the patients. Evaluation of phenotypes of strains. |
Heterogeneity of results. High NPV. Two positive consecutive results have high specificity and high positive likelihood ratio, single negative PCR result has high NPV. High sensitivity in combination with other fungal biomarkers in serum (either GM or BDG) or in BAL and along with GM and/or LFD test. |
Rapid. | Requires further clinical standardization. Potential for contamination due to the environmental ubiquity of fungal nucleic acids. |
| Aspergillus-specific immuno-chromatographic lateral flow device (LFD) test [36,49,51,63,66,74,83][4][6][11][25][28][36][45] | Acceptable sensitivity, specificity, moderate PPV, high NPV (especially in combination with BAL GM) [66,[2884]][46]. |
Rapid. | Requires further clinical evaluation. Sensitivity of the BAL LFD assay may be reduced in the presence of antifungal treatment. |
|
| Novel assays: volatile organic compounds (VOC) assays, Gliotoxin (GT), bis(methylthio)gliotoxin (bmGT) assays [67,68,85,86,87][29][30][47][48][49] |
High sensitivity and specificity. bmGT presents higher sensitivity and PPV than GM and similar specificity and NPV. Importantly, the combination of GM and bmGT increased the PPV (100%) and NPV (97.5%) of the individual biomarkers. |
Rapid. | Requires further clinical evaluation. |
CAPA: COVID-19-Associated Pulmonary Aspergillosis, GM: Galactomannan, BAL: Bronchoalveolar lavage, NPV: Negative Predictive Value, PPV: Positive Predictive Value, BDG: b-D-glucan, PCR: Polymerase Chain Reaction, LFD: Lateral Flow Device, VOC: Volatile Organic Compounds, GT: Gliotoxin, bmGT: bis(methylthio)gliotoxin.
2. The Role of Diagnostic Radiology
Differentiating between Aspergillus colonization and IPA is notoriously difficult, especially in the ICU setting. In the absence of host factors and diagnostic criteria, as defined by the EORTC, invasive or high-risk diagnostics (biopsy or CT scan) are required, to support the diagnosis of IPA [55][12]. However, the radiologic findings associated with IA are non-specific and often represent other IFIs such as mucormycosis or different nonfungal diseases, such as bacterial pneumonia, cryptogenic organizing pneumonitis (COP), or even hemorrhage [88][50].
Unarguably, the diagnostic process for CAPA should include Aspergillus antigen tests from serum and BAL, including enzyme-linked immunosorbent assay (ELISA), LFD, GM, or Aspergillus PCR, along with chest CT imaging, since nodules with halo sign or other characteristic features of IA on chest CT were seen in 17.6% of COVID-19 patients with severe disease, but was not confirmed to be IPA. This is in accordance with the absence of classic chest CT characteristics of IAPA. Consequently, the lack of typical features, such as cavities, should not exclude CAPA. On the other hand, the presence of such features should support the diagnosis and reduce the number of further laboratory examinations [35][3].
Due to severe life-threatening hypoxia and challenges in mechanical ventilation, CT scanning is not considered possible for many patients with SARS-CoV-2. When performed, the differentiation between COVID-19 and Aspergillus-associated lesions could additionally be proved extremely complex [34][19]. Moreover, patient transfer to CT in these cases is often resource intensive. Clinical justification of CT procedures should be made on a local level, and CT should be reserved for cases where healthcare team discussion highlights a clear clinical indication.
References
- Lamoth, F.; Lewis, R.E.; Walsh, T.J.; Kontoyiannis, D.P. Navigating the uncertainties of COVID-19 associated aspergillosis (CAPA): A comparison with influenza associated aspergillosis (IAPA). J. Infect. Dis. 2021, 26, 163.
- Verweij, P.E.; Rijnders, B.J.A.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T.; et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: An expert opinion. Intensive Care Med. 2020, 46, 1524–1535.
- Armstrong-James, D.; Youngs, J.; Bicanic, T.; Abdolrasouli, A.; Denning, D.W.; Johnson, E.; Mehra, V.; Pagliuca, T.; Patel, B.; Rhodes, J.; et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur. Respir. J. 2020, 56, 2002554.
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38.
- Hage, C.A.; Carmona, E.M.; Epelbaum, O.; Evans, S.E.; Gabe, L.M.; Haydour, Q.; Knox, K.S.; Kolls, J.K.; Murad, M.H.; Wengenack, N.L.; et al. Microbiological Laboratory Testing in the Diagnosis of Fungal Infections in Pulmonary and Critical Care Practice. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2019, 200, 535–550.
- Patterson, T.F.; Thompson, G.R. 3rd.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60.
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481.
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376.
- Blot, S.I.; Taccone, F.S.; Van den Abeele, A.M.; Bulpa, P.; Meersseman, W.; Brusselaers, N.; Dimopoulos, G.; Paiva, J.A.; Misset, B.; Rello, J.; et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 2012, 186, 56–64.
- Thornton, C.; Johnson, G.; Agrawal, S. Detection of invasive pulmonary aspergillosis in haematological malignancy patients by using lateral-flow technology. J. Vis. Exp. 2012, 61, 3721.
- Maertens, J.A.; Blennow, O.; Duarte, R.F.; Muñoz, P. The current management landscape: Aspergillosis. J. Antimicrob. Chemother. 2016, 71, ii23–ii29.
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821.
- Trof, R.J.; Beishuizen, A.; Debets-Ossenkopp, Y.J.; Girbes, A.R.; Groeneveld, A.B. Management of invasive pulmonary aspergillosis in non-neutropenic critically ill patients. Intensive Care Med. 2007, 33, 1694–1703.
- Hope, W.W.; Walsh, T.J.; Denning, D.W. Laboratory diagnosis of invasive aspergillosis. Lancet Infect. Dis. 2005, 5, 609–622.
- Thornton, C.R. Detection of invasive aspergillosis. Adv. Appl. Microbiol. 2010, 70, 187–216.
- Pickering, J.W.; Sant, H.W.; Bowles, C.A.P.; Roberts, W.L.; Woods, G.L. Evaluation of a (1->3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2005, 43, 5957–5962.
- Meersseman, W.; Lagrou, K.; Maertens, J.; Wilmer, A.; Hermans, G.; Vanderschueren, S.; Spriet, I.; Verbeken, E.; Van Wijngaerden, E. Galactomannan in bronchoalveolar lavage fluid: A tool for diagnosing aspergillosis in intensive care unit patients. Am. J. Respir. Crit. Care Med. 2008, 177, 27–34.
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792.
- Rutsaert, L.; Steinfort, N.; Van Hunsel, T.; Bomans, P.; Naesens, R.; Mertes, H.; Dits, H.; Van Regenmortel, N. COVID-19-associated invasive pulmonary aspergillosis. Ann. Intensive Care 2020, 10, 71.
- Koehler, P.; Cornely, O.A.; Böttiger, B.W.; Dusse, F.; Eichenauer, D.A.; Fuchs, F.; Hallek, M.; Jung, N.; Klein, F.; Persigehl, T.; et al. COVID-19 associated pulmonary aspergillosis. Mycoses 2020, 63, 528–534.
- Alanio, A.; Dellière, S.; Fodil, S.; Bretagne, S.; Mégarbane, B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir. Med. 2020, 8, e48–e49.
- Verweij, P.E.; Gangneux, J.P.; Bassetti, M.; Brüggemann, R.J.M.; Cornely, O.A.; Koehler, P.; Lass-Flörl, C.; van de Veerdonk, F.L.; Chakrabarti, A.; Hoenigl, M.; et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020, 1, e53–e55.
- Wiederhold, N.P.; Thornton, C.R.; Najvar, L.K.; Kirkpatrick, W.R.; Bocanegra, R.; Patterson, T.F. Comparison of Lateral Flow Technology and Galactomannan and (1→3)-β-d-Glucan Assays for Detection of Invasive Pulmonary Aspergillosis. Clin. Vaccine Immunol. 2009, 16, 1844–1846.
- Heldt, S.; Hoenigl, M. Lateral Flow Assays for the Diagnosis of Invasive Aspergillosis: Current Status. Curr. Fungal Infect. Rep. 2017, 11, 45–51.
- Hoenigl, M.; Eigl, S.; Heldt, S.; Duettmann, W.; Thornton, C.; Prattes, J. Clinical evaluation of the newly formatted lateral-flow device for invasive pulmonary aspergillosis. Mycoses 2018, 61, 40–43.
- Wahidi, M.M.; Lamb, C.; Murgu, S.; Musani, A.; Shojaee, S.; Sachdeva, A.; Maldonado, F.; Mahmood, K.; Kinsey, M.; Sethi, S.; et al. American Association for Bronchology and Interventional Pulmonology (AABIP) Statement on the Use of Bronchoscopy and Respiratory Specimen Collection in Patients with Suspected or Confirmed COVID-19 Infection. J. Bronchol. Interv. Pulmonol. 2020, 27, e52–e54.
- Kramer, R.; Abraham, W.R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37.
- Bassetti, M.; Peghin, M.; Vena, A. Challenges and Solution of Invasive Aspergillosis in Non-neutropenic Patients: A Review. Infect. Dis. Ther. 2018, 7, 17–27.
- Koo, S.; Thomas, H.R.; Daniels, S.D.; Lynch, R.C.; Fortier, S.M.; Shea, M.M.; Rearden, P.; Comolli, J.C.; Baden, L.R.; Marty, F.M. A Breath Fungal Secondary Metabolite Signature to Diagnose Invasive Aspergillosis. Clin. Infect. Dis. 2014, 59, 1733–1740.
- Mercier, T.; Reséndiz Sharpe, A.; Waumans, D.; Desmet, K.; Lagrou, K.; Maertens, J. Gliotoxin and bis(methylthio)gliotoxin are not reliable as biomarkers of invasive aspergillosis. Mycoses 2019, 62, 945–948.
- Zhou, W.; Li, H.; Zhang, Y.; Huang, M.; He, Q.; Li, P.; Zhang, F.; Shi, Y.; Su, X. Diagnostic Value of Galactomannan Antigen Test in Serum and Bronchoalveolar Lavage Fluid Samples from Patients with Nonneutropenic Invasive Pulmonary Aspergillosis. J. Clin. Microbiol. 2017, 55, 2153–2161.
- Rickerts, V.; Mousset, S.; Lambrecht, E.; Tintelnot, K.; Schwerdtfeger, R.; Presterl, E.; Jacobi, V.; Just-Nübling, G.; Bialek, R. Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin. Infect. Dis. 2007, 44, 1078–1083.
- Lewis, R.E.; Kontoyiannis, D.P. Invasive aspergillosis in glucocorticoid-treated patients. Med. Mycol. 2009, 47, S271–S281.
- Zou, M.; Tang, L.; Zhao, S.; Zhao, Z.; Chen, L.; Chen, P.; Huang, Z.; Li, J.; Chen, L.; Fan, X. Systematic review and meta-analysis of detecting galactomannan in bronchoalveolar lavage fluid for diagnosing invasive aspergillosis. PLoS ONE 2012, 7, e43347.
- Guo, Y.L.; Chen, Y.Q.; Wang, K.; Qin, S.M.; Wu, C.; Kong, J.L. Accuracy of BAL galactomannan in diagnosing invasive aspergillosis: A bivariate metaanalysis and systematic review. Chest 2010, 138, 817–824.
- Eigl, S.; Prattes, J.; Reinwald, M.; Thornton, C.R.; Reischies, F.; Spiess, B.; Neumeister, P.; Zollner-Schwetz, I.; Raggam, R.B.; Flick, H.; et al. Influence of mould-active antifungal treatment on the performance of the Aspergillus-specific bronchoalveolar lavage fluid lateral-flow device test. Int. J. Antimicrob. Agents 2015, 46, 401–405.
- De Heer, K.; Gerritsen, M.G.; Visser, C.E.; Leeflang, M.M. Galactomannan detection in broncho-alveolar lavage fluid for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst. Rev. 2019, 5, CD012399.
- Theel, E.S.; Jespersen, D.J.; Iqbal, S.; Bestrom, J.E.; Rollins, L.O.; Misner, L.J.; Markley, B.J.; Mandrekar, J.; Baddour, L.M.; Limper, A.H.; et al. Detection of (1, 3)-β-D-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia 2013, 175, 33–41.
- White, P.L.; Wiederhold, N.P.; Loeffler, J.; Najvar, L.K.; Melchers, W.; Herrera, M.; Bretagne, S.; Wickes, B.; Kirkpatrick, W.R.; Barnes, R.A.; et al. Comparison of Nonculture Blood-Based Tests for Diagnosing Invasive Aspergillosis in an Animal Model. J. Clin. Microbiol. 2016, 54, 960–966.
- White, P.L.; Wingard, J.R.; Bretagne, S.; Löffler, J.; Patterson, T.F.; Slavin, M.A.; Barnes, R.A.; Pappas, P.G.; Donnelly, J.P. Aspergillus Polymerase Chain Reaction: Systematic Review of Evidence for Clinical Use in Comparison with Antigen Testing. Clin. Infect. Dis. 2015, 61, 1293–1303.
- Arvanitis, M.; Ziakas, P.D.; Zacharioudakis, I.M.; Zervou, F.N.; Caliendo, A.M.; Mylonakis, E. PCR in diagnosis of invasive aspergillosis: A meta-analysis of diagnostic performance. J. Clin. Microbiol. 2014, 52, 3731–3742.
- Arvanitis, M.; Anagnostou, T.; Mylonakis, E. Galactomannan and Polymerase Chain Reaction-Based Screening for Invasive Aspergillosis Among High-Risk Hematology Patients: A Diagnostic Meta-analysis. Clin. Infect. Dis. 2015, 61, 1263–1272.
- Mengoli, C.; Cruciani, M.; Barnes, R.A.; Loeffler, J.; Donnelly, J.P. Use of PCR for diagnosis of invasive aspergillosis: Systematic review and meta-analysis. Lancet Infect. Dis. 2009, 9, 89–96.
- Hoenigl, M.; Prattes, J.; Spiess, B.; Wagner, J.; Prueller, F.; Raggam, R.B.; Posch, V.; Duettmann, W.; Hoenigl, K.; Wölfler, A.; et al. Performance of galactomannan, beta-d-glucan, Aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J. Clin. Microbiol. 2014, 52, 2039–2045.
- Pan, Z.; Fu, M.; Zhang, J.; Zhou, H.; Fu, Y.; Zhou, J. Diagnostic accuracy of a novel lateral-flow device in invasive aspergillosis: A meta-analysis. J. Med. Microbiol. 2015, 64, 702–707.
- Prattes, J.; Flick, H.; Prüller, F.; Koidl, C.; Raggam, R.B.; Palfner, M.; Eigl, S.; Buzina, W.; Zollner-Schwetz, I.; Thornton, C.R.; et al. Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am. J. Respir. Crit. Care Med. 2014, 190, 922–929.
- Acharige, M.J.T.; Koshy, S.; Ismail, N.; Aloum, O.; Jazaerly, M.; Astudillo, C.L.; Koo, S. Breath-based diagnosis of fungal infections. J. Breath Res. 2018, 12, 027108.
- Vidal-García, M.; Domingo, M.P.; De Rueda, B.; Roc, L.; Delgado, M.P.; Revillo, M.J.; Pardo, J.; Gálvez, E.M.; Rezusta, A. Clinical validity of bis(methylthio)gliotoxin for the diagnosis of invasive aspergillosis. Appl. Microbiol. Biotechnol. 2016, 100, 2327–2334.
- Vidal-García, M.; Sánchez-Chueca, P.; Domingo, M.P.; Ballester, C.; Roc, L.; Ferrer, I.; Revillo, M.J.; Pardo, J.; Gálvez, E.M.; Rezusta, A. Disseminated aspergillosis in an immunocompetent patient with detectable bis(methylthio)gliotoxin and negative galactomannan. Rev. Iberoam. Micol. 2017, 34, 49–52.
- Greene, R.E.; Schlamm, H.T.; Oestmann, J.W.; Stark, P.; Durand, C.; Lortholary, O.; Wingard, J.R.; Herbrecht, R.; Ribaud, P.; Patterson, T.F.; et al. Imaging findings in acute invasive pulmonary aspergillosis: Clinical significance of the halo sign. Clin. Infect. Dis. 2007, 44, 373–379.
More
