Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Dan Yaniv and Version 2 by Lindsay Dong.
Non-melanoma skin cancer (NMSC) is the most common malignancy in the United States. While surgery is considered as the main treatment modality for both cutaneous basal cell carcinoma (cBCC) and cutaneous squamous cell carcinoma (cSCC), radiotherapy plays an important role in the treatment of NMSC, both in the adjuvant setting for cases considered high-risk for recurrence, and in the definitive setting, when surgery is not feasible or desired by the patient.
- non-melanoma skin cancer
- cutaneous squamous cell carcinoma
- cutaneous basal cell carcinoma
- radiotherapy
1. Introduction
Non-melanoma skin cancer (NMSC) is the most common malignancy in the United States, accounting for over three million new cases each year. A total of 80% of them are basal cell carcinoma (BCC), 20% squamous cell carcinoma (SCC), and less than one percent other tumors such as Merkel cell carcinoma and adnexal tumors [1][2][1,2]. The risk factors for NMSC mainly include high cumulative ultra-violet radiation sun exposure [3][4][3,4], especially to the head and neck area; increasing age; immunosuppression—either drug-induced among organ transplant patients and cased by an autoimmune disease or due to hematologic malignancies, specifically chronic lymphocytic leukemia (CLL) or human immunodeficiency virus (HIV); chronic inflammation [5]; and certain genetic conditions such as Xeroderma Pigmentosum [6].
While the standard of care for curative intent therapy is surgical treatment for both cutaneous BCC (cBCC) and cutaneous SCC (cSCC) patients, radiotherapy plays a significant role both in the definitive and adjuvant settings [7].
In the definitive setting, radiotherapy is considered an accepted alternative approach to surgery among patients who are medically inoperable, those who refuse surgery, and in cases where surgical excision may be associated with a poor cosmetic outcome [8].
Different radiation methods—including Kilo-voltage (soft) X-rays, mega-voltage electrons, mega-voltage X-rays, and low dose rate (LDR)/high dose rate (HDR) interventional radiotherapy (brachytherapy) and proton therapy—are all accepted radiotherapy modalities for NMSC [9].
In the adjuvant setting, radiotherapy is considered by the National Comprehensive Cancer Network (NCCN) in cases of positive tumor margins after surgical resection among patients not amenable to re-excision, and in cases with high-risk features for tumor recurrence, such as peri-neural invasion (PNI), lympho-vascular invasion (LVI), head and neck location, ill-defined borders, a rapidly growing tumor, a tumor larger than two centimeters, deep tumor invasion beyond the dermis or into deep structures (i.e., bone invasion), specific histologic features such as acantholytic, adenosquamous, metaplastic, or desmoplastic subtypes, recurrent tumors, and tumors in immunosuppressed patients [10].
23. Radiotherapy Techniques for NMSC
2.1. Early-Stage Lesions
3.1. Early-Stage Lesions
In cutaneous lesions, a major challenge is achieving a therapeutic dose at the surface while targeting the tumor to its entire depth. Several methods may be employed:-
Soft X-ray (contact) therapy: This method entails placing a cone directly onto the irradiated surface, typically with the delivery of the dose at energies of 30–100 kV. This may target lesions up to 10 mm deep at a therapeutic dose. The advantages of this method include a low penumbral dose and easy clinical setup. Its major disadvantages, especially in the definitive setting, are unclarity regarding the subclinical spread, and technical difficulty in measuring the tumor depth. This method may be preferred in well-demarcated and well-palpable superficial and symmetrical lesions [11] (Figure 1).
-
Electron beam radiotherapy: This is delivered via a linear accelerator (LINAC) at energies of 6–20 MeV and may target superficial lesions, with a therapeutic depth of up to 5 cm (which is the depth of the 90% isodose line for 20 MeV). The setup may be clinical or assisted by computerized tomography (CT) simulation; beam collimation is performed by lead blocks (standard or personalized). This method is useful in treating relatively large fields and lesions deeper than 1 cm without compromising superficial tissues. Its disadvantages include a relatively large lateral spread (especially at higher energies), cumbersome beam collimation, and a skin sparing effect at 6 MeV [12] (Figure 2 and Figure 3).
-
Mega-Voltage (MV) photon beam therapy: This is delivered via a LINAC at energies of 6–18 MV. A clinical setup is possible, but treatment is mostly planned via CT simulation. This method allows for more sophisticated treatment planning (forward or inverse) and beam collimation, and higher certainty regarding the dose delivery. However, MV photons have a skin sparing effect, and therefore require a tissue compensator (bolus) to be placed on the superficial portion of the tumor, which may be technically challenging and reduce the aforementioned setup certainty. This is the preferred method for the treatment of tumors with a deep-set component or with proximity to critical structures [13] (Figure 4).
-
Interventional radiotherapy (brachytherapy): This is currently mostly performed with interstitial catheters and an HDR source, or by personalized surface molds. This method is useful for lesions with complex geometry, where external beam therapy may result in an inhomogeneous dose distribution, or in proximity to critical structures; this method may also be employed after failure of conventional EBRT due to the ability to deliver high doses per fraction with little collateral damage. While data regarding the specific protocols are limited, common protocols for HDR include 30–50 Gy given in 5–10 fractions [14].
-
Proton therapy: The use of this novel technique of charged particle therapy is becoming more and more widespread in routine practice, especially in the setting of re-irradiation. It is delivered via a particle accelerator and generally planned inversely. Its major advantage is the Bragg peak, which delivers a high dose to the target with a very rapid falloff, and therefore a very low exit dose. Its main disadvantages are the cost and scarce availability, as well as technical challenges regarding the entrance dose [15][15[16],16].
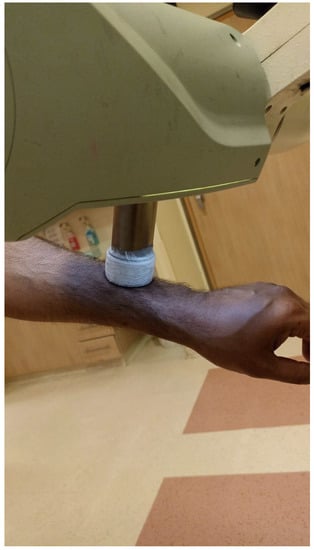
Figure 1.
Soft X-ray cone positioning.
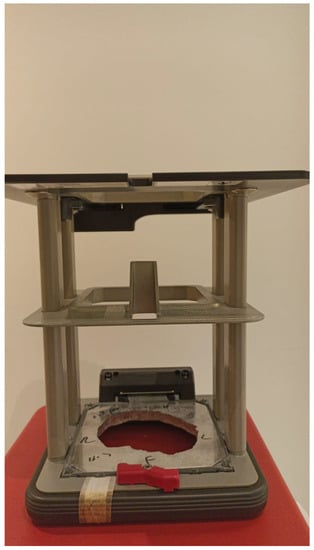
Figure 2.
Personalized lead block for electron treatment.
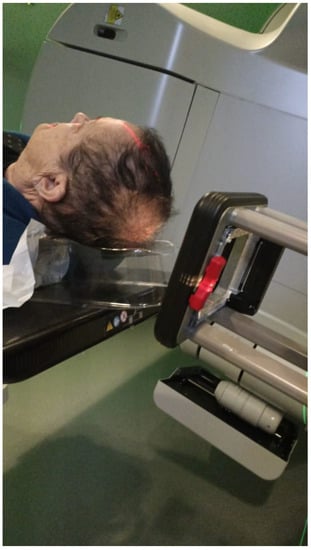
Figure 3.
Electron beam treatment.

Figure 4.
Thermoplastic mask with personalized bolus attached for MV photon treatment.
2.2. Advanced Lesions—Locally Advanced or Neck Involvement
3.2. Advanced Lesions—Locally Advanced or Neck Involvement
Radiotherapy to deep-set structures, whether in the definitive or the postoperative setting (as either adjuvant or salvage treatment), is usually performed by MV photon intensity modulated radiotherapy (IMRT) with strict immobilization of the patient (in the head and neck region, via a thermoplastic mask). This allows for the protection of normal structures while delivering a homogenous dose to the target.
An alternative is intensity modulated proton therapy, which may protect normal structures better and reduce the integral dose due to a very low exit dose [15].
2.3. Dose Fractionation Schemes
3.3. Dose Fractionation Schemes
A wide range of doses and fractionation schemes are utilized clinically, as is reflected in the NCCN guidelines, recommending that a biologic effective dose of 70–93 Gy is to be achieved in the definitive setting and 60–79 Gy in the adjuvant setting, referring to an alpha/beta ratio of 10 [10].
Hypofractionation may be useful for small-volume superficial lesions, where it may not result in severe toxicity [17][18][17,18]. The NCCN guidelines recommend a biologic effective dose of 56–88 Gy in the definitive setting, and 56–70 Gy in the adjuvant setting. Hypofractionation of large volumes (i.e., neck radiotherapy) is not formally recommended [10].
In early-stage lesions, the treated volume is typically the gross tumor with 0.3–0.5 cm of clinical target volume.
Elective neck irradiation: When treating locally advanced lesions with definitive or adjuvant intent, elective neck radiotherapy may be employed. Commonly, the radiation fields are large and may include (depending on the location of the primary) levels 2–4, the superficial lobe of the parotid gland, level 1B for lesions anterior to the ear, and level 5 for posterior lesions. Contralateral elective radiotherapy is generally not warranted [19].
34. Radiation as Primary Therapy with Curative Intent
3.1. Early-Stage cBCC and cSCC
4.1. Early-Stage cBCC and cSCC
A randomized trial by Avril et al. [20][23] compared surgical treatment with radiation treatment for patients with cutaneous BCC of the face measuring up to 4 cm. They were able to demonstrate a significantly higher rate of local control among the 174 patients treated with surgery compared with the 173 treated with radiation (Log rank p = 0.003). While 87% of patients treated with surgery reported a good cosmetic result, only 69% of the radiotherapy arm reported such result (p < 0.01).
3.2. Advanced Stage Non-Metastatic cSCC and cBCC
4.2. Advanced Stage Non-Metastatic cSCC and cBCC
Lee et al. [21][28] studied the role of radiotherapy as a definitive treatment modality among patients with advanced cSCC. They demonstrated a 53% initial 5-year control rate and 74% ultimate control rate (including patients with treatment failure successfully salvaged with surgery). Bone involvement, perineural invasion, and prior radiotherapy were all associated with a worse control rate. Al-Othman et al. [22][29] also evaluated the efficacy of definitive radiotherapy for advanced-stage NMSC, and found a 53% initial and 90% ultimate control rate, quite similar to the results of Lee. As the performance of CT simulation for treatment planning was not available during the time period described, the relatively low primary radiation treatment control rate described in the abovementioned papers is not surprising, as marginal failure was more common in those since CT simulation for treatment planning was not yet available.45. Adjuvant Treatment
4.1. Perineural Invasion
5.1. Perineural Invasion
PNI is more frequently seen in patients with cSCC (5% to 10%) than in patients with BCC (2% to 5%) [23][39]. In the case of clinical PNI (cPNI), the Trigeminal (V) and Facial (VII) cranial nerves (CN) are commonly affected, typically involving retrograde progression (progression of the disease from the periphery (skin) toward the brain/brainstem). PNI is considered a factor associated with a worse prognosis among cSCC, as it is associated with higher rates of local, regional, and distant recurrence [24][25][26][40,41,42]. Patients with asymptomatic microscopic PNI may benefit from local adjuvant radiotherapy over a wide field but do not necessarily require treatment to the entire course of the relevant cranial nerve [13].4.2. Regional Disease
5.2. Regional Disease
A study by Coombs et al. [27][49] reviewed 63 patients with metastatic cSCC to the parotid. All patients underwent surgical therapy that included parotidectomy with neck dissection. Fifty-one patients (81%) were treated with adjuvant radiotherapy. The five-year disease-specific survival was significantly improved among patients treated with adjuvant radiotherapy (84% vs. 48%, p = 0.0076). A similar study by Hirshoren et al. [28][50] also describes a series of 78 patients with parotid cSCC metastases. Sixty-five underwent superficial parotidectomy (83.3%) and 13 total parotidectomy (16.7%). Of note, the median age of the patients was 79 years. Sixty-four patients were treated with adjuvant radiotherapy (82.1%). The institute’s protocol for adjuvant radiation included 60 Gy in 30 fractions, or 66 Gy in 33 fractions in cases of extracapsular extension or positive margins.4.3. Immunosuppression
5.3. Immunosuppression
Immunosuppression is considered a risk factor for NMSC, with solid organ transplant recipients experiencing a 65- to 100-fold increase in the incidence of cSCC [29][30][31][32][52,53,54,55]. Furthermore, it seems that cSCC among this patient group is more aggressive in nature, with a higher rate of locoregional recurrence and distant metastases [33][34][35][56,57,58]. CLL patients are also prone to a more aggressive course of cSCC [36][59]. Kadakia et al. [37][60] evaluated the role of adjuvant radiation among 53 immunocompromised patients with scalp SCC. Thirty-one were solid organ transplant recipients, 19 had CLL, and 3 had HIV. On pathology, 69.8% had PNI, 24.4% had LVI, and 39.6% had a poorly differentiated tumor. All patients were referred to adjuvant radiation therapy. However, only 45 complied to the recommendation and 8 did not receive the treatment. The three-year DFS among patients who received adjuvant radiation was 80% compared to 62.5% among patients who were treated by surgery alone, and the OS was 62% vs. 37.5% (significance not mentioned).56. Radiotherapy Combined with Chemotherapy in the Definitive/Adjuvant Setting for Locally Advanced cSCC
There is no high-level randomized controlled evidence in support of the addition of chemotherapy to radiotherapy for locally advanced cSCC. However, this is a common practice, due to the inherent chemosensitivity of these tumors and as an extrapolation from mucosal head and neck SCC. In the adjuvant setting, recent randomized data are available. TROG 05.01 randomized 321 patients receiving 60–66 Gy of adjuvant radiotherapy in addition to weekly carboplatin or radiotherapy only. This trial demonstrated no benefit from the addition of chemotherapy [38][64]. However, single-agent carboplatin is not an accepted radiosensitizing regimen, and the cohort may be too small to demonstrate a significant difference. Another prospective trial compared weekly cetuximab with radiotherapy to historical data with a favorable local control profile, with a cost of about 15% grade 3 toxicity [39][65]. Retrospective data did show a recurrence-free (although not overall) survival advantage for the addition of platinum-based chemotherapy to adjuvant radiotherapy with high-risk lesions (multiple involved nodes, ECE, or involved margins, as an extrapolation from mucosal head and neck SCC) [40][66].67. Elective Neck Irradiation
The role of elective neck treatment in head and neck cSCC has been a matter of debate [41][42][43][68,69,70]. The first question in order is whether elective treatment—either surgical or by irradiation—is at all necessary among cSCC patients. Amit et al. attempted to examine the role of surgical treatment for cSCC, by describing 1111 patients with head and neck cSCC. Of them, 173 (16%) underwent elective neck dissection, and the rest were observed and surgically salvaged if regional disease appeared. They found no survival benefit among patients treated electively compared with those who were observed and salvaged when cervical metastases appeared. While the overall rate of occult regional spread among those treated electively was 21% (36 of 173), only 5% of patients treated by observation experienced regional recurrence (49 of 938). A multivariate analysis did not demonstrate a survival benefit for the performance of elective neck dissection. These results demonstrate that elective neck treatment is not necessarily associated with survival benefit and should be considered on a case-to-case basis.78. Radiation and Immunotherapy
Immune checkpoint inhibitors, especially anti-programmed cell death-1 receptor (anti-PD1) antibodies such as pembrolizumab and cemiplimab, have been approved for the treatment of cSCC not amenable for curative intent treatment with tremendous results [44][73], and have been evaluated for BCC as a second line in patients who develop resistance to Hedgehog pathway inhibitors [45][74]. Still, the response to immunotherapy is seen in around 50% of patients [46][75]. Radiotherapy has a direct effect on tumoral cells because it induces cellular DNA damage. However, in recent years more evidence was gathered on the immunogenic effect of radiation. The damage radiation induces in cancer cells can convert them into an in-situ tumor vaccine by inducing the release of neoantigens during cancer cell death in association with proinflammatory signals that trigger the innate immune system to activate tumor-specific T cells. This is mediated by three important signals: calreticulin, high-mobility group box-1 (HMGP-1), and adenosine triphosphate (ATP) [47][76]. In addition, radiation’s effects on the tumor microenvironment enhance the infiltration of activated T cells and can overcome some of the barriers to tumor recognition by the immune system. The translocation of calreticulin and other signals to the cell surface promotes the uptake of dying cancer cells by dendritic cells and the release of antigens that can be efficiently presented [48][77], which could allow for responses in distant nonirradiated sites, namely the abscopal effect [49][78].89. NMSC Radiation Toxicity
8.1. Early-Stage Lesions
9.1. Early-Stage Lesions
1. Acute toxicity—due to the superficial nature of radiotherapy on the one hand, and the targeting of the skin on the other, the main acute toxicity is radiation dermatitis, which may amount to grade 3 toxicity (i.e., wet desquamation). It is typically treated with topical agents and is usually short-lived due to the quick proliferation of cutaneous keratinocytes [50][80]. 2. Late toxicity—the main concern is usually skin necrosis, especially in poorly vascularized areas (scalp, shin), or around skin flaps that have not completely healed at the initiation of radiotherapy. Other concerns are hair loss at the irradiated area, discoloration, and chondronecrosis (mainly in ear or nose lesions). Many papers describing radiotherapy for early-stage lesions do not expressly report late toxicity rates. Those that do range between 0 and 5.7% (for radiotherapy to the pinna) [50][80].8.2. Advanced-Stage Lesions
9.2. Advanced-Stage Lesions
As mentioned above, in advanced-stage lesions—whether treated with definitive or adjuvant intent—a larger and deeper volume is typically irradiated many times with elective treatment to the neck nodal basin and parotid, and therefore the toxicity profile is different. However, mainly due to the unilaterality of radiotherapy and sparing of mucosa, the rate of grade 3–5 adverse events is very low, as little as 0% in some series [51][72]. However, concurrent systemic therapy (platinum-based or off-label cetuximab) may increase acute dermatitis and mucositis to up to 40% [40][52][66,82].-
Acute toxicity—If the skin is targeted, dermatitis may occur as described above. Elective radiotherapy to the neck may result in mucositis and dysphagia. Radiotherapy to the parotid and course of the facial nerve may result in xerostomia and metallic taste.
-
Late toxicity—While dermatitis and mucositis usually resolve, chronic xerostomia may occur. Tinnitus may occur if the base of the skull is irradiated. Thyroiditis may occur for lower neck radiotherapy, and the acceleration of carotid artery stenosis may also occur.
