Chromium coating is a vital process for many industries, such as the aerospace or the automotive sectors. This is due to the interesting properties that those types of coatings bring: reducing friction and increasing hardness, as well as wear and corrosion resistance
[1]. As a consequence, this treatment allows one to improve the life time of the pieces coated as well, making the pieces more reliable when in use
[2]. However, the most common technology used to make those types of coatings is chromium electrodeposition. That technology is quite popular due to its advantages of having low investment and consumable costs, ease of implementation, and relatively low energy consumption compared to other coating technologies. However, one of the drawbacks of the technology is its use of hexavalent chromium compounds, which have a strong carcinogenic effect and have been shown to increase the risks of lung cancer in the workers of electrodeposition plants
[3]. This is due to the emission of droplets during the electrodeposition process caused by bubble formation with the electrolysis of the plating solution. Furthermore, the supply chain of the chromium compounds used in the electrodeposition process is also responsible for a lot of hexavalent chromium emissions along the chain
[4]. Hexavalent chromium has been infamously present in a number of environmental and health cases, the most famous of which has been adapted to the big screen in the movie Erin Brockovich and involves the contamination of groundwater near Hinkley, California in 1993 by the Pacific Gas and Electric company, which has led to inhabitants developing cancer
[5]. Additionally, installations of chromium electroplating have been demonstrated to lead to soil contamination in the surrounding area
[6]. There is then a pressure on industry to either invest large amounts of money in preventive measures to abate the hexavalent chromium emissions, or to use alternative technologies to chromium electrodeposition
[7]. Indeed, while currently chromium trioxide is used in the EU at an annual rate of 7000 tons, the use of this compound is technically forbidden unless an authorization is granted by the European Commission. Such authorizations have been given for electroplating industries, but are set to expire in September 2024
[8]. While these authorizations can be renewed, the European Parliament took legal action against the European Commission over granting those authorizations
[9]. This makes the future of chromium electroplating uncertain in the EU and might make alternatives to the process a necessity rather than a preference. As such, it is required to assess those alternatives on an economical and environmental basis to evaluate their viability.
2. State-of-the-Art
2.1. Thermal Spraying and HVOF
Thermal spraying is a family of deposition techniques that relies on the projection of heated material towards a substrate in order to grow a coating. Thermal spraying was first developed in 1910
[14][15], starting with flame spraying in its original form, which involves the combustion of fuel gases to generate heat for melting a feed of particles, as well as expanding gases that will give speed to the melted particles. Those temperatures are usually around 2700 °C, and particle speeds reach up to 100 m/s
[15][16]. Since then, the newer variants of the technology still use the same basic principles, but now they require more advanced hardware. Thermal spraying technologies, which derive from flame spraying are: Detonation Gun (D-Gun) spraying, High-Velocity Oxy-Fuel spraying (HVOF), and High-Velocity Air–Fuel spraying (HVAF).
With detonation gun spraying, fuel gases and oxygen are confined in a tube, and the detonation is initiated using a spark. A high-pressure shockwave is then generated, and the droplets can be sent at higher temperatures (4000 °C) and much higher speeds (up to 1200 m/s) than with typical flame spraying. The detonation cycle can happen between 1 and 10 times per second, and thanks to the increased temperatures and speeds, better adherence and lower porosities than flame spraying coatings can be achieved
[16][17][17,18].
With HVOF, the process relies on the combustion of a pressurized mixture of oxygen and fuel, which both come in a continuous gas flow. With a high flowrate and pressure, high temperature and supersonic speeds can be achieved (3000 °C and 1000 m/s respectively)
[18][19]. Similarly to D-Gun spraying, good adherence and low porosity can be achieved compared to conventional flame spraying. HVOF comes in addition with the ability to attain higher deposition rates thanks to the continuous nature of the process. HVOF is usually performed with powders, but solutions and suspensions can be used instead of powders
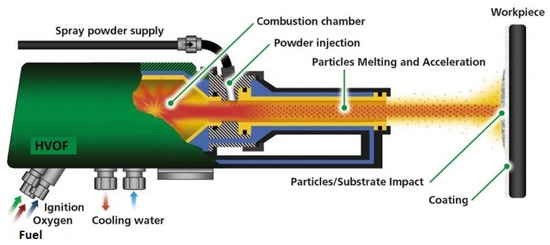
[19][20]. A representation of a HVOF gun can be seen in
Figure 1.
Figure 1.
Representation of the HVOF process, credit to Flame Spray Technologies (FST).
HVAF is extremely similar to HVOF, except that oxygen is replaced with air. This has the advantage of being cheaper than HVOF, but due to the lower oxygen content, lower temperatures can be attained and so the range of materials HVAF can treat is smaller
[20][21].
Other thermal spraying methods exist that do not involve combustion in the process. They can broadly be put into two categories: plasma spraying and kinetic spraying. Plasma spraying can involve different technologies, which each use plasma as an energy source. First, Atmospheric Plasma Spraying (APS) takes place at atmospheric pressure and uses a discharge to generate the plasma. The use of a plasma jet allows to reach much higher temperatures for the melt that is being projected on the substrate, being in the range of 10,000 to 30,000 °C
[21][22], increasing the range of materials that can be melted. Second, solution or suspension precursor plasma spraying (SPS/SPPS), where the same principle is used, except the feed is in liquid state and finer powders can be used. Third, low-pressure or vacuum plasma spraying where the process takes place in a vacuum chamber and the “in-flight” interactions of the materials are limited. Additionally, finally, wire arc spraying, where a wire is fed to the process and its material is melted through the generation of an electrical arc, is observed.
Kinetic spraying, also called cold spraying, does not involve extensive heating of the feed, but instead relies on the transfer of large amounts of kinetic energy to the material in order obtain the conditions necessary to grow the coating. The gas used is itself hot. However, the particles remain in a solid state, rather than being melted. For these types of coatings, the feedstock has to be limited to certain materials that can meet the conditions necessary to be deposited in that state. However, the low temperatures allow lower residual stresses, as well as lower amounts of oxidation of the material during the deposition
[22][23]. Typical temperatures for the gas are 400 °C to 800 °C, while, depending on the material and other factors, velocities can range from a few hundred m/s to 1400 m/s for their upper limit
[23][24].
As it stands, HVOF is one of the technologies most regarded as being a potential replacement to hard chromium deposition. The reasons for this will be developed further in the next section, and other potential replacements for the hexavalent chromium will also be discussed in a further section. On a technical side, HVOF has the capacity to deposit hard coatings, such as tungsten carbide in a cobalt matrix (from hereon called WC/Co), at high deposition speeds. Those coatings are often compared to hard chromium coatings due to their high performance in terms of hardness, toughness, and wear resistance
[24][25], which is on par with, if not higher than, chromium coatings. Indeed, superior wear resistance has often been demonstrated in the literature of WC/Co compared to hard chromium
[25][26][27][26,27,28]. Furthermore, as HVOF is not capable of depositing pure chromium due to its oxidation temperature
[28][29], other types of coatings have to be studied when considering HVOF. However, there might be some aspects that hold back HVOF as a technology. For instance, the investment costs are higher compared to electrodeposition, and the combustion of fuel and subsequent CO
2 emissions add to the operational costs and environmental impact of the HVOF process. Additionally, HVOF cannot be used for some pieces, such as tubes, due to the requirement of a line of sight from the gun to the substrate. Finally, in the case of HVOF deposition of WC/Co, other additional challenges are faced as well: for example, the high amounts of energy are required to make tungsten carbides or the use of cobalt, a rare and toxic metal. All of these factors can make the deposition of WC/Co with HVOF a less attractive process. An overview of the assessments already achieved in the literature will be presented. The goal of that overview is to obtain some insight about the HVOF process in order to identify the different upsides and downsides, both economic and environmental, of WC/Co deposited by HVOF compared to electrodeposition.
2.2. Previous Economic and Environmental Assessment of the HVOF Technology
As said previously, HVOF is widely regarded as a good option to replace chromium electrodeposition and is then relatively often evaluated based on its economic and environmental performances, even though rarely on both aspects simultaneously. For example, in the ESTCP Cost and Performance Report
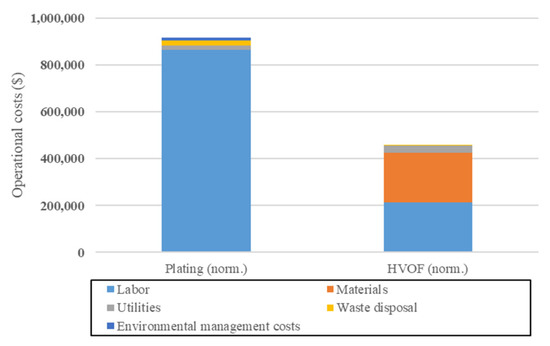
[29][30], Sartwell et al. aimed to assess, among other aspects, the operational costs associated with a partial switch from chromium electrodeposition to HVOF. The results obtained are quite encouraging for HVOF, showing a reduction of about 50% in the yearly operational costs compared to electrodeposition, as shown in
Figure 2.
Figure 2. Comparisons of the yearly operational costs of HVOF and chromium electrodeposition. Adapted from [29]. Comparisons of the yearly operational costs of HVOF and chromium electrodeposition. Adapted from [30].
The main cost reduction comes from the reduction in labor cost, which shows about a four-fold reduction. Furthermore, although they are not very important, HVOF eliminates most costs associated to waste disposal (treatment of spent solution) and environmental management (abatement of emissions) that are present in Chromium electrodeposition processes. However, the materials costs increased by a wide margin, being about as costly as labor. This is due to the WC/Co powder used for HVOF being much more expensive compared to the reactants used in chromium electrodeposition. It should be noted that the yearly production volume for both technologies is uneven in the original publication, i.e., 65% of the yearly production was considered to be handled by HVOF, while 35% was considered to be handled by chromium electrodeposition. In
Figure 2, annual costs have been linearly scaled to represent an annual production volume of 5500 pieces for each technology.
In another publication, from Krishnan et al., the environmental performances of HVOF and electrodeposition for landing gears have been compared
[30][31]. LCA has been used for the analysis, and the results for the endpoints have been compared. Impacts for HVOF in the ‘Human Health’ and ‘Ecosystem Quality’ categories are clearly lower than for the electrodeposition process (between a factor 2 and 10 for ‘Human Health’ depending on the case, and several degrees of magnitude for ‘Ecosystem Quality’). This is due mostly to the toxicity of the compounds used in the electrodeposition process. On the other hand, results are more mixed for the ‘Resources’ category, being relatively equal for both processes, but for different reasons: the impacts for electrodeposition are mainly driven by electricity consumption, while the impacts of HVOF are mainly driven by powder production.
In another publication from Parker et al.
[31][32], better mechanical performances were found for HVOF compared to electrodeposition, as well as better corrosion resistance in the atmospheric exposition test. However, corrosion resistance was equivalent for both technologies in the ASTM B117 test. Furthermore, a reduction in operational costs of about 20% was found with the usage of HVOF. This is a figure quite different from the 50% reduction obtained in
[29][30]. However, a 50% reduction can also be obtained in
[31][32], provided the stripping phase (removal of the old coating from the piece before the application of a new coating) for HVOF is not included, as is the case in
[31][32].
Finally, in other publications from Sartwell et al.
[32][33][33,34], HVOF coatings have been found to be better in terms of fatigue and leakage protection. Furthermore, HVOF has shown better corrosion resistance compared to electrodeposition. Additionally, finally, a much lower requirement of labor time was found compared to electrodeposition, explaining the labor cost reductions obtained in the previous studies.
As hard chromium plating has been developed for close to 100 years at the time of writing, with the first chromium coatings dating from the 1920′s
[34][35], it is undeniably more mature than HVOF. Indeed, HVOF has only seen first experiments from the 1980s onwards
[35][36]. This leads to HVOF competing with a more settled technology, such as hard chromium. That being said, HVOF has the potential to replace chromium electrodeposition for given applications. This is thanks to its novel ability to deposit materials, such as ceramics, at very high deposition speeds. To evaluate that potential, its performance needs to be evaluated.
2.3. Other Potential Technologies for the Replacement of Hexavalent Chromium
Finally, it should be noted that HVOF is not the only technology well regarded for the replacement of hexavalent chromium electrodeposition. Many technologies have also been investigated during the years
[36][37][37,38], with some of them being, in the same manner as HVOF, not reliant on a liquid medium, such as: magnetron sputtering
[38][39], arc deposition
[39][40], laser cladding
[40][41], ion beam
[41][42], or chemical vapor deposition
[42][43]. All of these technologies have the capacity to produce coatings with interesting properties that allow them to be able to replace hexavalent chromium plating in some fashion, with high hardnesses and high wear resistances that allow them to take the place of chromium to coat newly produced pieces. In a lot of cases, however, there are some aspects of the technologies that hold them back as full replacement of hexavalent chromium. For example, with magnetron sputtering and other similar physical vapor technologies, large coating thicknesses cannot be realistically reached, and those technologies cannot, therefore, replace chromium plating for the purpose of coatings designed for the repair of damaged equipment. Other types of drawbacks can include, for example, coatings providing similar or better wear resistance, but not providing enough corrosion resistance, or requiring high temperatures for the growth of the coating, which can limit the choice of the substrate.
Another technology among the most promising to replace hexavalent chromium electrodeposition is trivalent chromium electrodeposition. Indeed, by replacing the hexavalent chromium salts with a non-toxic variant, the health problems can be totally avoided, all while keeping a relatively similar process. Using such types of baths, the use of lead anodes was not required, and large thicknesses of hundreds of micrometres have been reached, with similar or higher deposition rate as hexavalent solutions, as well as with similar or better hardness
[43][44][45][44,45,46]. While such advantages would make trivalent chromium a strict upgrade to hexavalent chromium, unfortunately the process has some drawbacks, which render its adoption and implementation in the industry difficult. Those drawbacks include: the high voltage potential required for the reduction of Cr
+3 to the metallic state, the stability of trivalent chromium compounds in aqueous solutions, and the quick precipitation of chromium hydroxides from the solution
[46][47]. These drawbacks imply the requirement of a strict control of the composition and acidity of the bath where complexing agents have to be used in order to lower the potential of Cr
+3 reduction and prevent the formation of stable compounds and of hydroxides. Furthermore, the coating obtained with trivalent plating is an alloy of chromium (for example, Cr-C), rather than pure metallic chromium, which can alter its properties, and the bath may then require additives, for example, to change the coloration of the coating. Therefore, the practicality of making thick coatings at a large scale using trivalent chromium can be compromised, as well as its ability to make repairs of damaged pieces. Nevertheless, trivalent chromium plating’s potential as a replacement to hexavalent chromium should not be underestimated, and it deserves its own sustainability assessment study.