Neurofilament light chain (NfL) is a neuron specific structural protein which can be detected in the blood (serum and plasma). Elevate blood levels may serve as an important surrogate neuro-axonal injury in a variety of neurological conditions, already showing promising associations with outcomes of interest.
- biomarker
- blood
- neurofilament light chain
- pathophysiology
- multiple sclerosis
1. Neurofilament Structure and Function
Neurofilaments are neuronal-specific heteropolymers conventionally considered to consist of a triplet of light (NfL), medium (NfM) and heavy (NfH) chains according to their molecular mass [1]. More recent discoveries show that α-Internexin in the central nervous system [2] and peripherin in the peripheral nervous system [3] can also be included in neurofilament heteropolymers. These five proteins co-assemble into the 10 nM intermediate filaments in different combinations and concentrations depending on the type of neuron, location in the axon and stage of development [4].
Neurofilaments are neuronal-specific heteropolymers conventionally considered to consist of a triplet of light (NfL), medium (NfM) and heavy (NfH) chains according to their molecular mass [1]. More recent discoveries show that α-Internexin in the central nervous system [2] and peripherin in the peripheral nervous system [3] can also be included in neurofilament heteropolymers. These five proteins co-assemble into the 10 nM intermediate filaments in different combinations and concentrations depending on the type of neuron, location in the axon and stage of development [4].
Each of the neurofilament proteins consists of an amino-terminal domain that is thought to regulate the formation of oligomers [5], a central helical rod domain, and a variable carboxy-terminal domain. The chain-specific C-terminal domains are the main determinants of differences in molecular mass and phosphorylation between subunits. Following synthesis and assembly in the neuron cell body, tetramers of neurofilament proteins are transported bidirectionally along axons by the microtubular apparatus prior to forming a continuously overlapping array that runs parallel to axons. Once formed, in the healthy state, they are remarkably stable for months to years [6].
In mature myelinated axons, neurofilaments are the single most abundant protein [7]. They perform key roles as part of the neuroaxonal scaffold to resist external pressures, determine axonal diameter, indirectly moderate conduction velocity, and act as an attachment for organelles and other proteins [4]. Beyond their primary structural role in axons, mounting evidence indicates that a unique pool of synaptic neurofilament proteins serves dynamic functions beyond static structural support [8]. Changes in neurofilament phosphorylation may be involved in long term potentiation that underpins memory [9] and NMDA receptor stability is dependent on a synaptic scaffold of neurofilament proteins.
In mature myelinated axons, neurofilaments are the single most abundant protein [7]. They perform key roles as part of the neuroaxonal scaffold to resist external pressures, determine axonal diameter, indirectly moderate conduction velocity, and act as an attachment for organelles and other proteins [4]. Beyond their primary structural role in axons, mounting evidence indicates that a unique pool of synaptic neurofilament proteins serves dynamic functions beyond static structural support [8]. Changes in neurofilament phosphorylation may be involved in long term potentiation that underpins memory [9] and NMDA receptor stability is dependent on a synaptic scaffold of neurofilament proteins.
2. Neurofilament Pathophysiology
Figure 1, where we focus on NfL). Physiologic degradation of neurofilaments within neurons is proposed to be a combination of ubiquitin-mediated proteasomal and apophagocytotic pathways [10]. Based on the trafficking of other proteins degraded in the CNS, it is likely that partially degraded fragments of neurofilaments drain directly into CSF and blood via multiple routes. These include direct drainage into CSF and blood via arachnoid granulations as well as lymphatic drainage into the subarachnoid spaces and perivascular spaces [11][12]. Several studies have demonstrated strong correlations between blood and CSF NfL, with r values typically ranging from 0.7 to 8 (e.g., [13]). However, our understanding of the kinetics of neurofilament release, distribution and metabolism is incomplete.
, where we focus on NfL). Physiologic degradation of neurofilaments within neurons is proposed to be a combination of ubiquitin-mediated proteasomal and apophagocytotic pathways [10]. Based on the trafficking of other proteins degraded in the CNS, it is likely that partially degraded fragments of neurofilaments drain directly into CSF and blood via multiple routes. These include direct drainage into CSF and blood via arachnoid granulations as well as lymphatic drainage into the subarachnoid spaces and perivascular spaces [11,12]. Several studies have demonstrated strong correlations between blood and CSF NfL, with r values typically ranging from 0.7 to 8 (e.g., [13]). However, our understanding of the kinetics of neurofilament release, distribution and metabolism is incomplete.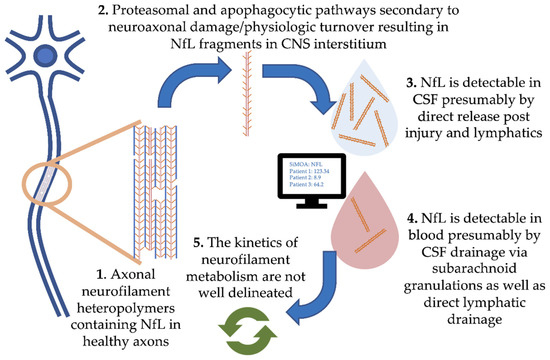
Figure 1.
Blood–brain barrier permeability itself may be a confounder; neurofilament quotient in blood compared to CSF could be selectively increased following periods of inflammation such as that seen in MS relapse, positively skewing blood NfL levels. Two recent studies on this topic in MS patients present conflicting results [14][15].
Blood–brain barrier permeability itself may be a confounder; neurofilament quotient in blood compared to CSF could be selectively increased following periods of inflammation such as that seen in MS relapse, positively skewing blood NfL levels. Two recent studies on this topic in MS patients present conflicting results [14,15]. Once NfL enters the blood, the half-life is a key consideration with implications on the frequency of disease activity monitoring. In a longitudinal study of NfL levels before and after intrathecal catheter insertion, NfL in both CSF and serum peaked at 1-month post-surgery, returning to baseline after 6 to 9 months [16]. In longitudinally sampled MS patients around the time of relapse, levels increasing 5 months before, peaking at clinical onset, and recovery within 4–5 months [17]. Therefore, quarterly measurement is likely sufficient, a frequency that our group is currently investigating in longitudinal prospective studies of serum NfL.Age is the principal physiologic covariate of NfL levels. Levels in healthy controls increase by 2.2% per year [18][19]. Furthermore, an inflection point is observable above the age of 60, after which both sNfL levels, as well as the inter-individual variability in levels, increase greatly [20]. It is speculated that these changes are attributable to both aging itself as well as the accumulation of subclinical comorbidities. Other factors outside of neurological disease itself that may alter neurofilament levels include BMI [21] as well as vascular risk factors [22]
Age is the principal physiologic covariate of NfL levels. Levels in healthy controls increase by 2.2% per year [18,19]. Furthermore, an inflection point is observable above the age of 60, after which both sNfL levels, as well as the inter-individual variability in levels, increase greatly [20]. It is speculated that these changes are attributable to both aging itself as well as the accumulation of subclinical comorbidities. Other factors outside of neurological disease itself that may alter neurofilament levels include BMI [21] as well as vascular risk factors [22].Although the primary focus of this review is the pathophysiologic relevance of NfL concentrations as they relate to neurological diseases such as MS, the vital role of neurofilaments is underlined by various human mutations that interfere with their function and homeostasis. Mutations of gigaxonin, a key component in the ubiquitin-dependent intermediate filament degradation, results in the pathological aggregation of neurofilaments in neurons and a severe neurodegenerative condition called giant axonal neuropathy [23]. Mutations in the neurofilament light chain gene itself result in axonal forms of hereditary motor sensory polyneuropathy [24] and variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis [25].
Although the primary focus of this review is the pathophysiologic relevance of NfL concentrations as they relate to neurological diseases such as MS, the vital role of neurofilaments is underlined by various human mutations that interfere with their function and homeostasis. Mutations of gigaxonin, a key component in the ubiquitin-dependent intermediate filament degradation, results in the pathological aggregation of neurofilaments in neurons and a severe neurodegenerative condition called giant axonal neuropathy [23]. Mutations in the neurofilament light chain gene itself result in axonal forms of hereditary motor sensory polyneuropathy [24] and variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis [25].3. Measurement of Blood Levels of Neurofilament Light Chain
3. Measurement of Blood Levels of Neurofilament Light Chain
Of the family of neurofilament proteins, neurofilament light chain (NfL) has gained the most interest as a candidate marker of outcomes in neurological diseases. This was not without contention. While the neurofilament light chain is the most abundant and soluble of the neurofilament proteins, phosphorylated neurofilament heavy chain (NfH) was initially thought to be more resistant to protease activity [31,32,33]. NfL was thought to be unstable in vitro [34] and initial research focused on NfH quantified by enzyme-linked immunosorbent assay (ELISA) or electrochemiluminescence (ECL) as a biomarker of axonal damage in MS [35,36]. However, in 2013, a comparative study of NfL and NfH found both proteins showed equivalent stability after several days at room temperature and through freeze–thaw cycles [37]. Moreover, although some of the differences observed may correspond to analytical methodologies, this study found that NfL levels were higher than NfH and NfL was a better discriminator of MS patients from controls.
Of the family of neurofilament proteins, neurofilament light chain (NfL) has gained the most interest as a candidate marker of outcomes in neurological diseases. This was not without contention. While the neurofilament light chain is the most abundant and soluble of the neurofilament proteins, phosphorylated neurofilament heavy chain (NfH) was initially thought to be more resistant to protease activity [26][27][28]. NfL was thought to be unstable in vitro [29] and initial research focused on NfH quantified by enzyme-linked immunosorbent assay (ELISA) or electrochemiluminescence (ECL) as a biomarker of axonal damage in MS [30][31]. However, in 2013, a comparative study of NfL and NfH found both proteins showed equivalent stability after several days at room temperature and through freeze–thaw cycles [32]. Moreover, although some of the differences observed may correspond to analytical methodologies, this study found that NfL levels were higher than NfH and NfL was a better discriminator of MS patients from controls.
Initial studies looking at NfL in association with neurological disease outcomes focused on CSF measurements. Although CSF is “closer” to the CNS pathologies (e.g., MS) and NfL concentration is approximately 500-fold higher, the inconvenient and invasive lumbar puncture required severely limits its clinical utility as a frequent serial biomarker. Concentrations in the blood however were too low to be reliably measured with conventional immunoassays such as ELISA or ECL assays. It was not until recently, with the development of the Single-Molecule Assay (SiMoA), that analytical methods become sufficiently sensitive to measure the single-digit picogram per milliliter levels present in blood [38]. This SiMoA technology, similar to other immunoassays, is based on fluorescent microbeads coated with high-affinity capture antibodies that bind NfL followed secondly by a fluorescently labelled detector antibody [39]. The increased analytical sensitivity of the SiMoA assay is due to its unique method of detection. Assay beads with captured NfL and fluorescent detector antibody, are loaded onto an assay disk containing >200,000 microwells capable of holding only a single bead. At high analyte concentration, the total fluorescence can be captured in the traditional manner (analog) and correlated to the analyte concentration. At low analyte concentrations, rather than detecting total fluorescence, a digital image is captured that enumerates individual fluorescent microwells in a binary fashion, effectively lowering the limit of quantitation to the femtomolar range. Although there are several neurofilament assays in development based on other technologies, including widely used chemiluminescent-based assay [40], the SiMoA platform has enabled most of the most recent insights.
Initial studies looking at NfL in association with neurological disease outcomes focused on CSF measurements. Although CSF is “closer” to the CNS pathologies (e.g., MS) and NfL concentration is approximately 500-fold higher, the inconvenient and invasive lumbar puncture required severely limits its clinical utility as a frequent serial biomarker. Concentrations in the blood however were too low to be reliably measured with conventional immunoassays such as ELISA or ECL assays. It was not until recently, with the development of the Single-Molecule Assay (SiMoA), that analytical methods become sufficiently sensitive to measure the single-digit picogram per milliliter levels present in blood [33]. This SiMoA technology, similar to other immunoassays, is based on fluorescent microbeads coated with high-affinity capture antibodies that bind NfL followed secondly by a fluorescently labelled detector antibody [34]. The increased analytical sensitivity of the SiMoA assay is due to its unique method of detection. Assay beads with captured NfL and fluorescent detector antibody, are loaded onto an assay disk containing >200,000 microwells capable of holding only a single bead. At high analyte concentration, the total fluorescence can be captured in the traditional manner (analog) and correlated to the analyte concentration. At low analyte concentrations, rather than detecting total fluorescence, a digital image is captured that enumerates individual fluorescent microwells in a binary fashion, effectively lowering the limit of quantitation to the femtomolar range. Although there are several neurofilament assays in development based on other technologies, including widely used chemiluminescent-based assay [35], the SiMoA platform has enabled most of the most recent insights.
3. Assocaitions between Blood Neurofilament Levels and Neurological Diseases
Measurement of a convenient objective blood marker of neuronal injury in patients is an appealing prospect for neurologists. Analogous to the cardiologist’s troponin, neurofilament light chain can be detected in the blood at elevated levels in a variety of neurological disease state in the central and peripheral nervous system causing neuroaxonal injury and can be followed longitudinally. These include neurodegenerative conditions, stroke, traumatic brain injury, peripheral demyelination and multiple sclerosis
[361][372]. Although not generally considered a marker of diagnostic utility, levels in amyotrophic lateral sclerosis (ALS) are so discrepantly elevated that they may be useful in this setting
[383]. In many of these conditions
[394], the current rate of neuronal loss is predictive of future losses, and as such NfL may be of prognostic utility. NfL has been most intensively studied in patients suffering from Multiple Sclerosis. Here there is a great unmet need for improved prediction and disease monitoring largely due to availability of a range disease modifying treatments which when employed appropriately can greatly reduce the morbidity associated with this condition. Many are optimistic that NfL could represent the first of its kind in neurology: a broadly-applicable protein biomarker that objectively reflects underlying pathology which can be harnessed to improve patient outcomes. Important future work includes the establishment of age-adjusted normative datasets and cutoff values so that physicians can better interpret individual patient results.
References
- Fuchs, E.; Cleveland, D.W. A structural scaffolding of intermediate filaments in health and disease. Science 1998, 279, 514–519.Michael Khalil; Charlotte E. Teunissen; Markus Otto; Fredrik Piehl; Maria Pia Sormani; Thomas Gattringer; Christian Barro; Ludwig Kappos; Manuel Comabella; Franz Fazekas; et al.Axel PetzoldKaj BlennowHenrik ZetterbergJens Kuhle Neurofilaments as biomarkers in neurological disorders. Nature Reviews Neurology 2018, 14, 577-589, 10.1038/s41582-018-0058-z.
- Yuan, A.; Rao, M.V.; Sasaki, T.; Chen, Y.; Kumar, A.; Veeranna; Liem, R.K.H.; Eyer, J.; Peterson, A.C.; Julien, J.P.; et al. α-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J. Neurosci. 2006, 26, 10006–10019.Christian Barro; Tanuja Chitnis; Howard L. Weiner; Blood neurofilament light: a critical review of its application to neurologic disease. Annals of Clinical and Translational Neurology 2020, 1, 1, 10.1002/acn3.51234.
- Yuan, A.; Sasaki, T.; Kumar, A.; Peterhoff, C.M.; Rao, M.V.; Liem, R.K.; Julien, J.P.; Nixon, R.A. Peripherin is a subunit of peripheral nerve neurofilaments: Implications for differential vulnerability of cns and peripheral nervous system axons. J. Neurosci. 2012, 32, 8501–8508.Lauren M. Forgrave; Matthew Ma; John R. Best; Mari L. Demarco; The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer's disease, frontotemporal dementia, and amyotrophic lateral sclerosis: A systematic review and meta‐analysis. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring 2019, 11, 730-743, 10.1016/j.dadm.2019.08.009.
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309.Bénédicte Delcoigne; Ali Manouchehrinia; Christian Barro; Pascal Benkert; Zuzanna Michalak; Ludwig Kappos; David Leppert; Jon A. Tsai; Tatiana Plavina; Bernd C. Kieseier; et al.Jan LyckeLars AlfredssonIngrid KockumJens KuhleTomas OlssonFredrik Piehl Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology 2020, 94, e1201-e1212, 10.1212/wnl.0000000000009097.
- Ching, G.Y.; Liem, R.K.H. Roles of head and tail domains in α-internexin’s self-assembly and coassembly with the neurofilament triplet proteins. J. Cell Sci. 1998, 111, 321–333.
- Millecamps, S.; Gowing, G.; Corti, O.; Mallet, J.; Julien, J.P. Conditional NF-L transgene expression in mice for in vivo analysis of turnover and transport rate of neurofilaments. J. Neurosci. 2007, 27, 4947–4956.
- Fliegner, K.H.; Liem, R.K.H. Cellular and Molecular Biology of Neuronal Intermediate Filaments. Int. Rev. Cytol. 1991, 131, 109–167.
- Bragina, L.; Conti, F. Expression of Neurofilament Subunits at Neocortical Glutamatergic and GABAergic Synapses. Front. Neuroanat. 2018, 12, 74.
- Hashimoto, R.; Nakamura, Y.; Komai, S.; Kashiwagi, Y.; Tamura, K.; Goto, T.; Aimoto, S.; Kaibuchi, K.; Shiosaka, S.; Takeda, M. Site-specific phosphorylation of neurofilament-L is mediated by calcium/calmodulin-dependent protein kinase II in the apical dendrites during long-term potentiation. J. Neurochem. 2000, 75, 373–382.
- Bomont, P. Degradation of the Intermediate Filament Family by Gigaxonin. Methods Enzymol. 2016, 569, 215–231.
- Carare, R.O.; Bernardes-Silva, M.; Newman, T.A.; Page, A.M.; Nicoll, J.A.R.; Perry, V.H.; Weller, R.O. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 2008, 34, 131–144.
- Gafson, A.R.; Barthélemy, N.R.; Bomont, P.; Carare, R.O.; Durham, H.D.; Julien, J.-P.; Kuhle, J.; Leppert, D.; Nixon, R.A.; Weller, R.O.; et al. Neurofilaments: Neurobiological foundations for biomarker applications. Brain 2020, 143, 1975–1998.
- Thebault, S.; Tessier, D.; Lee, H.; Bowman, M.; Bar-Or, A.; Arnold, D.L.; Atkins, H.; Tabard-Cossa, V.; Freedman, M.S. High Serum Neurofilament Light Chain normalises after Haematopoietic Stem Cell Transplant for MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e598.
- Kalm, M.; Boström, M.; Sandelius, Å.; Eriksson, Y.; Ek, C.J.; Blennow, K.; Björk-Eriksson, T.; Zetterberg, H. Serum concentrations of the axonal injury marker neurofilament light protein are not influenced by blood-brain barrier permeability. Brain Res. 2017, 1668, 12–19.
- Uher, T.; McComb, M.; Galkin, S.; Srpova, B.; Oechtering, J.; Barro, C.; Tyblova, M.; Bergsland, N.; Krasensky, J.; Dwyer, M.; et al. Neurofilament levels are associated with blood–brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult. Scler. J. 2020, 1–12.
- Bergman, J.; Dring, A.; Zetterberg, H.; Blennow, K.; Norgren, N.; Gilthorpe, J.; Bergenheim, T.; Svenningsson, A. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e271.
- Akgün, K.; Kretschmann, N.; Haase, R.; Proschmann, U.; Kitzler, H.H.; Reichmann, H.; Ziemssen, T. Profiling individual clinical responses by high-frequency serum neurofilament assessment in MS. Neurol. Neuroimmunol. NeuroInflamm. 2019, 6, 1–12.
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schädelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017, 81, 857–870.
- Barro, C.; Benkert, P.; Disanto, G.; Tsagkas, C.; Amann, M.; Naegelin, Y.; Leppert, D.; Gobbi, C.; Granziera, C.; Yaldizli, Ö.; et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018, 141, 2382–2391.
- Khalil, M.; Pirpamer, L.; Hofer, E.; Voortman, M.M.; Barro, C.; Leppert, D.; Benkert, P.; Ropele, S.; Enzinger, C.; Fazekas, F.; et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat. Commun. 2020, 11, 1–9.
- Manouchehrinia, A.; Piehl, F.; Hillert, J.; Kuhle, J.; Alfredsson, L.; Olsson, T.; Kockum, I. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann. Clin. Transl. Neurol. 2020, 7, 139–143.
- Korley, F.K.; Goldstick, J.; Mastali, M.; Van Eyk, J.E.; Barsan, W.; Meurer, W.J.; Sussman, J.; Falk, H.; Levine, D. Serum NfL (Neurofilament Light Chain) Levels and Incident Stroke in Adults with Diabetes Mellitus. Stroke 2019, 50, 1669–1675.
- Bomont, P.; Cavalier, L.; Blondeau, F.; Hamida, C. Ben; Belal, S.; Tazir, M.; Demir, E.; Topaloglu, H.; Korinthenberg, R.; Tüysüz, B.; et al. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat. Genet. 2000, 26, 370–374.
- Mersiyanova, I.V.; Perepelov, A.V.; Polyakov, A.V.; Sitnikov, V.F.; Dadali, E.L.; Oparin, R.B.; Petrin, A.N.; Evgrafov, O.V. A new variant of Charcot-Marie-Tooth disease type 2 is probably the result of a mutation in the neurofilament-light gene. Am. J. Hum. Genet. 2000, 67, 37–46.
- Figlewicz, D.A.; Krizus, A.; Martinoli, M.G.; Meininger, V.; Dib, M.; Rouleau, G.A.; Julien, J.P. Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum. Mol. Genet. 1994, 3, 1757–1761.
- Schlaepfer, W.W.; Lee, C.; Lee, V.M.; Zimmerman, U.-J.P. An Immunoblot Study of Neurofilament Degradation In Situ and During Calcium-Activated Proteolysis. J. Neurochem. 1985, 44, 502–509.
- Goldstein, M.E.; Sternberger, N.H.; Sternberger, L.A. Phosphorylation protects neurofilaments against proteolysis. J. Neuroimmunol. 1987, 14, 149–160.
- Pant, H.C. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem. J. 1988, 256, 665–668.
- Koel-Simmelink, M.J.A.; Teunissen, C.E.; Behradkia, P.; Blankenstein, M.A.; Petzold, A. The neurofilament light chain is not stable in vitro. Ann. Neurol. 2011, 69, 1065–1066.
- Petzold, A.; Keir, G.; Green, A.J.E.; Giovannoni, G.; Thompson, E.J. A specific ELISA for measuring neurofilament heavy chain phosphoforms. J. Immunol. Methods 2003, 278, 179–190.
- Leppert, D.; Petzold, A.; Regeniter, A.; Schindler, C.; Mehling, M.; Anthony, D.C.; Kappos, L.; Lindberg, R.L.P. Neurofilament heavy chain in CSF correlates with relapses and disability in multiple sclerosis. Neurology 2011, 76, 1206–1213.
- Kuhle, J.; Plattner, K.; Bestwick, J.P.; Lindberg, R.L.; Ramagopalan, S.V.; Norgren, N.; Nissim, A.; Malaspina, A.; Leppert, D.; Giovannoni, G.; et al. A comparative study of CSF neurofilament light and heavy chain protein in MS. Mult. Scler. J. 2013, 19, 1597–1603.
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, Å.; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016, 54, 1655–1661.
- Wilson, D.H.; Rissin, D.M.; Kan, C.W.; Fournier, D.R.; Piech, T.; Campbell, T.G.; Meyer, R.E.; Fishburn, M.W.; Cabrera, C.; Patel, P.P.; et al. The Simoa HD-1 Analyzer: A Novel Fully Automated Digital Immunoassay Analyzer with Single-Molecule Sensitivity and Multiplexing. J. Lab. Autom. 2016, 21, 533–547.
- Carvalho, T. Siemens Healthineers, Novartis Partner to Develop NfL Diagnostic Test. 2020. Available online: https://multiplesclerosisnewstoday.com/news-posts/2020/09/21/siemens-healthineers-novartis-partner-to-develop-new-nfl-diagnostic-test-for-ms/ (accessed on 28 October 2020).
- Michael Khalil; Charlotte E. Teunissen; Markus Otto; Fredrik Piehl; Maria Pia Sormani; Thomas Gattringer; Christian Barro; Ludwig Kappos; Manuel Comabella; Franz Fazekas; et al. Neurofilaments as biomarkers in neurological disorders. Nature Reviews Neurology 2018, 14, 577-589, 10.1038/s41582-018-0058-z.
- Christian Barro; Tanuja Chitnis; Howard L. Weiner; Blood neurofilament light: a critical review of its application to neurologic disease. Annals of Clinical and Translational Neurology 2020, 1, 1, 10.1002/acn3.51234.
- Lauren M. Forgrave; Matthew Ma; John R. Best; Mari L. Demarco; The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer's disease, frontotemporal dementia, and amyotrophic lateral sclerosis: A systematic review and meta‐analysis. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring 2019, 11, 730-743, 10.1016/j.dadm.2019.08.009.
- Bénédicte Delcoigne; Ali Manouchehrinia; Christian Barro; Pascal Benkert; Zuzanna Michalak; Ludwig Kappos; David Leppert; Jon A. Tsai; Tatiana Plavina; Bernd C. Kieseier; et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology 2020, 94, e1201-e1212, 10.1212/wnl.0000000000009097.
