Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Magnus Norgren.
In the history of cellulose chemistry, hydrogen bonding has been the predominant explanation when discussing intermolecular interactions between cellulose polymers. This is unfortunately the general consensus in scholarly textbooks and in many research articles, and it applies to several other biomacromolecules’ interactions as well. The amphiphilicity of cellulose and many other biopolymers, and thereby hydrophobic interactions, has to be taken into account to get a more complete picture.
- cellulose
- amphiphilicity
- intermolecular interactions
1. Introduction
In 2010, a paper by Lindman, Karlström and Stigsson discussed the mechanisms of cellulose dissolution, pointing out some discrepancies in the common literature, where hydrogen bonding was argued as the most crucial interaction to overcome. The paper (re)introduced hydrophobic interactions and cellulose amphiphilicity as essential to consider for the successful development of future cellulose solvents [1]. In a review by Medronho and Lindman et al., published two years later, cellulose solubility or insolubility in water was revisited more carefully [2]. By considering some fundamental polymer physicochemical principles and some widely recognized inconsistencies in cellulose’s behavior, the authors emphasized that the hydrophobic molecular interactions have been underestimated, relative to hydrogen bonding, and are significantly important for the understanding of cellulose. Cellulose amphiphilicity was highlighted again. This time, one of the journal editors brought the matter into the spotlight for serious scrutinization by the scientific community. In a follow-up response paper, published in the same journal issue, several well-reputed cellulose scientists with a wide range of experience and representing a variety of scientific disciplines were invited to debate what was coined as “the Lindman hypothesis” [3]. Since then, a myriad of studies have been published dealing with cellulose dissolution and regeneration, and the consequences of cellulose’s amphiphilicity.
2. The “History” of Cellulose in Science and Technology
2.1. Addressing Cellulose Intermolecular Interactions
2.1.1. In Cellulose Biosynthesis
Via freeze-fracture techniques applied in electron microscopy studies, cellulose biosynthesis by plasma membrane-bound complexes has been visualized. The first observations of the synthesis machinery were made at the tip of elongating cellulose microfibrils in the green alga Oocystis, and, due to this, the synthesis machinery was designated as the terminal complex (TC) [4]. In most algae, the TCs are organized as linear arrays, but in higher plants, the cellulose-synthesizing machinery is designated as rosettes and occurs in the form of hexagonal structures with six-fold symmetry [5,6][5][6]. Cellulose biosynthesis is a complex, cell-directed event. The nature of the enzyme complexes controls the outcome, essentially via the number and positioning of the glycosyltransferases within the complexes [7]. This thereafter leads to cellulose secretion to the cell surface through complex secretion pore structures.
Polymerization and crystallization are two separate and sequential steps in native cellulose biosynthesis [8]. It is known that the crystallization step is the rate-limiting step, and when crystallization is prevented by the binding of fluorescent brighteners, the polymerization rate can significantly increase. In biosynthesis, cellulose crystallization has been described as a three-step process, starting with the formation of monomolecular glucan chain sheets due to van der Waals forces. Thereafter, the association of these sheets leads to mini-crystals (sub-elementary fibrils) stabilized by H-bonding, and finally the convergence of the mini-crystals into the native crystalline microfibril takes place [9,10,11][9][10][11].
2.1.2. In Dissolution and Regeneration of Cellulose
Currently, two processes in the dissolution and regeneration of cellulose are of technical and commercial importance: the viscose process and the lyocell process. The manufacturing of viscose rayon from cellulose raw material is based on an invention by Cross, Bevan and Beadle in 1891. The treatment involves dissolving pulps (sulfite and prehydrolysis kraft pulps) or cotton with concentrated alkali (NaOH), followed by a reaction with carbon disulfide (xanthation), which makes the intermediate soluble in NaOH (aq) and possible to process by spinning, casting and regeneration into fibers (rayon) and films (cellophane). Another type of regenerated cellulose fiber is lyocell (U.S. brand name Tencel). In the lyocell process, the cellulose solution is produced from dissolving pulp using the N-methylmorpholine-N-oxide (NMMO) solvent. The lyocell fiber is precipitated from NMMO, in which no substitution of the hydroxyl groups occurs, and no chemical intermediates are formed. The invention appeared first in a patent in 1981 by McCorsley, describing the basic process of dissolving cellulose [12]. Nowadays, Lenzing is the world’s largest lyocell fiber manufacturer, capable of supplying ca. 130,000 metric tons of lyocell fiber for the global rayon market each year.
Cellulose is insoluble in water and in hydrocarbons, but soluble in several simple and “exotic” solvents. Thus, non-derivatizing solvents for cellulose show enormous variation, including, e.g., strongly acidic and alkaline aqueous systems; aqueous NaOH and urea; transition metal complexes, such as copper salts mixed with concentrated ammonia (Cuoxam); aqueous copper–ethylenediamine complex solutions (CED); mixtures of dimethylsulfoxide with metal salts; N,N-dimethylacetamide (DMAc)/LiCl, NMMO; concentrated ZnCl2 aqueous solutions; and a range of different ionic liquids (IL). In recent reviews, many additional examples are given and here also the significant diversity of solvents is underlined [13,14][13][14]. From a thermodynamic point of view, the formation of extended crystalline regions in cellulose implies the lowered solubility of these regions compared with the amorphous ones, since the crystalline state always has the lowest free energy.
2.1.3. In Cellulose Swelling
Due to their complex hierarchical structures and organization, cellulose fibers show a different picture, characterized by heterogeneous swelling and dissolution [16][15]. The heterogeneous swelling can elicit unusual effects, such as the ballooning that occurs due to preferential swelling in specific areas along the fibers (Figure 21). In 1864, Nägeli described the ballooning phenomenon [17][16], and it was further reported in investigations by Pennetier [18][17], Flemming and Thaysen [19[18][19],20], Rollins and Tripp [21][20], Hock [22][21] and Warwicker et al. [23][22]. Later studies have shown that the swelling and dissolution mechanisms are strongly coupled with the solvent quality [24,25][23][24].
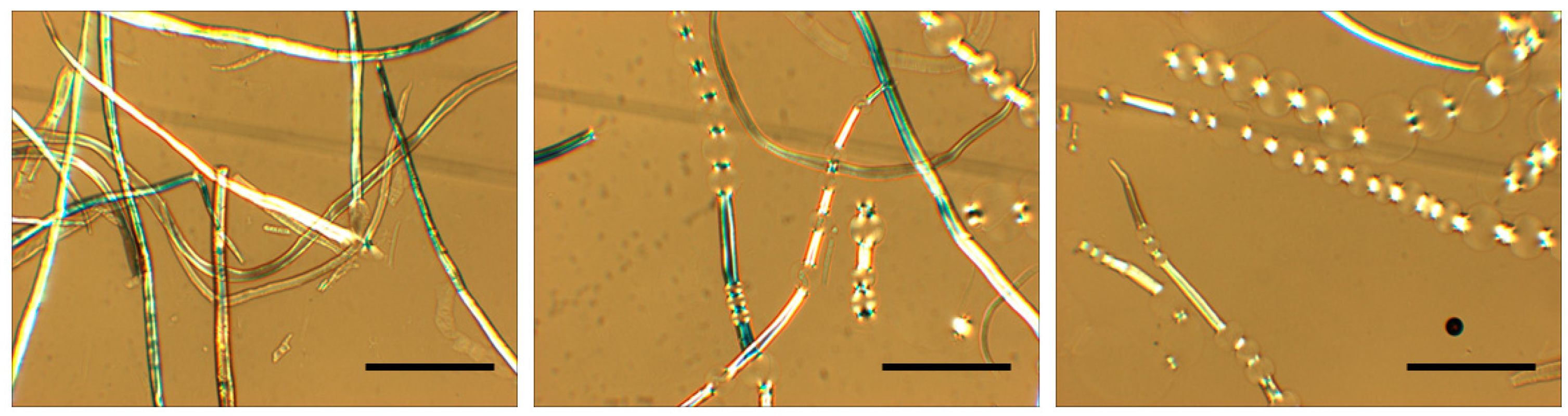
Figure 21. Nonhomogeneous swelling (ballooning phenomenon) in cellulose fibers when dispersed in cold aqueous-based alkali. The scale bar represents 100 μm.
As described above, cellulose swelling in strongly alkaline solutions has been known for a long time, and the swelling is often accompanied, to some extent, by dissolution. In the groundbreaking work by Neale, this was attributed to the osmotic pressure of the counterions, as cellulose is deprotonated and charged at a high pH [26][25]. It was further discovered that, along with swelling at high hydroxide concentrations, there might be appreciable swelling also at a lower pH due to the occurrence of acidic groups, especially carboxylic acid groups, formed during wood pulp processing through hydrogen peroxide bleaching [27,28][26][27]. Moreover, sulfonic acid groups from the chemi-thermomechanical or sulfite pulping processes may also contribute to this. Furthermore, Lindström and Carlsson noted that the water retention values of holocellulose and unbleached sulfate pulps showed major increases as a function of pH, in the range in which carboxylic acid groups ionize [27][26]. Clearly, the osmotic swelling due to counterion entropy, notably in polymer gels, is due to the ionization of the polysaccharides and lignin, e.g., due to the dissociation of carboxylic acid groups in cellulose and hemicelluloses (and sulfonic acid and/or phenolic groups in lignin). As for polymer systems in general, swelling increases with the charge density and decreases with the electrolyte concentration and the valency of the counterions [29,30][28][29].
Regarding the combination of hydrophobic and electrostatic interactions in cellulose, the addition of thiourea produces enhanced cellulose swelling in NaOH solutions, as was reported by Zhang et al. [31][30]. Strong effects of electrolyte additions, where the difference in polarity between the two ions is significant, can also be expected to promote swelling—namely, combinations of high-charge-density cations, such as Ca2+ and Li+, with large, polarizable anions, such as I− and SCN−. For example, LiSCN is very effective in enhancing swelling [32][31], which can be interpreted as the weak association of the anions with cellulose, whereas the cations are depleted. Furthermore, the significant swelling of cellulose fibers has been demonstrated using mixed solutions of NaSCN and urea [16,33][15][32].
2.1.4. In Partial Dissolution and Plasticization of Cellulose
Plasticization can in some ways be regarded as extreme swelling, gelation or the partial dissolution of cellulose fibers, which drastically increases the cellulose chain’s mobility. Efficient plasticizing solvents should have similar properties to good dissolving agents. In this respect, the early findings of urea as a plasticizing agent were vital, showing that the weakening of the hydrophobic interactions between cellulose molecules has a key role [34][33]. Plasticized, or vulcanized, paper was already developed in the 1860s, when several layers of paper, impregnated by zinc chloride, capable of swelling the cellulose fibers and partially dissolving them, were pressed together and zinc chloride washed out in several steps [35][34]. Plasticization increases the density, mechanical strength and strain at the break of the paper, with an unchanged or slightly increased specific stiffness [36,37][35][36]. With improved mechanical properties, the range of uses for cellulose fiber products is expanded, capable of replacing plastics in many applications.
Plasticization partly changes the crystallinity of cellulose fibers from cellulose I to cellulose II and increases the content of amorphous cellulose, which thereby influences the properties of the fibers and paper [38][37]. Furthermore, plasticization increases the fiber-to-fiber bond strength. The surface layers of the cellulose fibers can also be affected, and higher strain at break, stiffness and tensile strength can be introduced [39,40,41][38][39][40].
2.1.5. In Cellulose Pulp Fiber and Papermaking
Since ancient times, paper has been produced from fibers that were initially obtained via the processing of annual plants. Thanks to cellulose, correctly processed plants hold the fibrous structure needed to form a sufficiently strong network that can be dewatered, pressed and finally dried into sheets for further utilization. In the early days, the mechanical processing of plant raw material into lignocellulosic pulp, mainly for use as printing paper, was the only alternative. During industrialization in the 19th century, the mechanical processing of wood into fibers by “disassembling” logs with a stone grinder, often driven by waterpower, into groundwood pulp was introduced. Slightly later, chemical pulping was invented and evolved rapidly.
In its dried state, wood consists of polysaccharides and polyaromatic lignin, giving the wood a yellowish to brownish color. Somewhat dependent on the wood species, about 70% of the dry weight is constituted by the polysaccharides, and hereof cellulose is the main component [42][41]. In chemical pulping, most of the lignin is removed from the fibers and the superior brightness stability of the paper can be obtained after subsequent bleaching. The removal of lignin from the cellulose pulp fibers also makes them more flexible and adaptable when forming a web, and the produced paper material generally becomes much stronger. Thus, delignification brought new possibilities to utilize cellulose fibers in areas beyond printing paper, which were later exploited during the 20th century.
Due to the versatility of paper and its economic importance for many countries, a respectable amount of academic and industrial research has been dedicated to the topic over the years. A significant focus has been placed on developing and understanding the paper strength and the influence of fiber–water interactions [43][42]. The strength of the paper is obviously necessary for the final product, but, often, it is even more critical in paper production. The production rate in a paper machine can be far above 1000 m/min, and the forces exhibited under production can easily be detrimental and result in paper breaks and lost production. Of course, the different unit operations in the paper production process must be optimized as well. However, the paper strength from the wet to the dry state is finally the limiting parameter.
There is no doubt that hydrogen bonding between the fibers in paper has some importance for the strength, especially when no chemical additives are added into the papermaking stock. However, the beneficial effects of hydrogen bonding in the fiber–fiber interactions and paper strength are strictly limited to dried paper. If water surrounds the fibers, other phenomena due to, e.g., morphological changes related to fibrillation, and the geometrical and mechanical properties of the fibers and fiber walls introduced by directed processing, are always much more relevant to discuss [43][42]. Unfortunately, very often in scholarly textbooks and research literature discussing papermaking, hydrogen bonding has been incorrectly used as a simple explanation for fiber–fiber interactions and paper strength. In a very recent review by Wohlert et al., an excellent overview was presented of the current knowledge of intermolecular interactions related to cellulose-based materials at different hierarchical scales, from oligomers to macroscopic fibers [44][43].
2.2. Revisiting Cellulose—The Lindman Hypothesis
2.2.1. Considerations and Implications of Cellulose’s Dual Properties
The insolubility of cellulose in water is, in many publications, considered as a result of the complex hydrogen bonding network [45,46][44][45]. Since the general view of cellulose in the past suggested the dissolution of the polymer by breaking the cellulose–cellulose hydrogen bonds, it implied that the key to increasing cellulose’s solubility is to find a solvent that effectively disrupts the interchain hydrogen bonding in cellulose. Thus, the argued explanation for cellulose solubility in certain solvents, such as ILs, is that they “break” the hydrogen bonds. Moreover, in a somewhat imprecise way, some authors also refer to the crystallinity of cellulose as a contributing factor in its insolubility [47,48][46][47]. In general, polymer solubility is dependent on a balance between entropy, which drives solubility, and enthalpy, which opposes solubility. Entropy contributions are of different types, such as translational (determined by polymer molecular weight), configurational (determined by polymer flexibility) and counterion entropy (ionic polymers). Therefore, low-molecular-weight polymers are more soluble than high-molecular-weight ones, flexible ones are more soluble than stiff ones and ionic ones are more soluble than non-ionic ones.
Regarding the interactions determining the enthalpy, the strong hydrogen bonding between cellulose molecules has been emphasized in the literature. It is true that there are strong hydrogen bonds between cellulose molecules, but it has often been forgotten that, on dissolution in water, these are replaced by cellulose–water hydrogen bonds, which are equally as strong (ca. 5 kcal/mole) [49][48]. Hydrogen bonding can, therefore, not explain the low aqueous solubility. In fact, solute–solute hydrogen bonding in an aqueous solution is not expected to drive association or cause insolubility. Water is a highly hydrogen-bonded solvent. On the introduction of a solute, there is the formation of “cavities” in water. If the solute is nonpolar, there is a large opposing force for solubility, since cavity formation leads to a loss of water–water hydrogen bonding. There is partial compensation, especially at lower temperatures, because of the “structuring” of water around the co-solute; this leads to anomalously high solubility at low temperatures, as clearly manifested in the non-monotonic variation in many properties around room temperature [50][49]. On the association of two nonpolar molecules in water, “hydrophobic association”, there is a reversal of this process and a considerable gain in free energy due to the release of water molecules to the bulk (increasing the entropic contribution) and formation of water–water hydrogen bonds.
If cellulose is argued to be amphiphilic, its solubility and association behavior in water should be significantly affected by co-solutes known to eliminate/weaken hydrophobic interactions. In this respect, urea is well known to eliminate hydrophobic association in protein denaturation and surfactant demicellization, and surfactants have significant effects on cellulose solubility [46,51,52,53][45][50][51][52]. A general property of amphiphilic compounds is to migrate to interfaces, a tendency particularly observable in aqueous systems [54][53]. Further consequences are to self-assemble—for many polymers, this results in in gel formation—and to associate with other amphiphilic compounds, such as surfactants, polar lipids and block copolymers.
Close examination of the crystal structure of cellulose reveals that there is clear spatial segregation of C-H and O-H bonds, thus leading to both nonpolar and polar regions and suggesting distinct amphiphilicity [55,56,57][54][55][56]. It can be noted that other polyglucoses, such as cyclodextrins and amylose, also display distinctly nonpolar domains and interact strongly with both polar and nonpolar molecules [58][57].
2.2.2. Response from the Scientific Community on Lindman’s Dispute
The Lindman hypothesis [2] was debated by some of the most well-known cellulose scientists in a follow-up article [3]. The overall conclusion from his peers can be summarized by the following quote: “The general perspective of cellulose as a polymer in which intermolecular stress transfer involves more than hydrogen bonds. Hydrophobic and amphiphilic behaviors have been acknowledged for some time but may have been underestimated in conventional considerations of structure, solubility, etc. Ever since the discovery of hydrogen bonds, there has been a tendency to over-exaggerate their importance in determining the solid-state structure. The energy of a hydrogen bond is much more than the van der Waals energy of attraction between say C–H groups, but we must remember that there are a lot of C–H groups in cellulose.”
From the biosynthesis perspective, if it takes place in discrete steps in time and space, then these findings could reflect the structural inhomogeneities, which could lead to better understand how to formulate more efficient cellulose solvents. Some other compounds, e.g., carboxymethylcellulose, have a very distinct and dramatic role, inhibiting higher-order aggregations in cellulose’s ribbon structure [59][58]. This would indicate that native agents co-secreted with cellulose could change and control cellulose’s crystallinity. Since living organisms create cellulose structures that are in fact different on some structural level, while being identical in chemical (molecular) structure, the properties and possible dissolution behavior would be different. The latter might be a consequence of the thermodynamically driven molecular aggregation process of cellulose chains, being influenced by the presence of heteropolysaccharides and proteins during the biosynthesis.
References
- Lindman, B.; Karlström, G.; Stigsson, L. On the Mechanism of Dissolution of Cellulose. J. Mol. Liq. 2010, 156, 76–81.
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing Cellulose (in) Solubility: Reviewing Basic Physicochemical Aspects and Role of Hydrophobic Interactions. Cellulose 2012, 19, 581–587.
- Glasser, W.G.; Atalla, R.H.; Blackwell, J.; Malcolm Brown, R.; Burchard, W.; French, A.D.; Klemm, D.O.; Nishiyama, Y. About the Structure of Cellulose: Debating the Lindman Hypothesis. Cellulose 2012, 19, 589–598.
- Brown, R.M.; Montezinos, D. Cellulose Microfibrils: Visualization of Biosynthetic and Orienting Complexes in Association with the Plasma Membrane. Proc. Natl. Acad. Sci. USA 1976, 73, 143–147.
- Giddings, T.H.; Brower, D.L.; Staehelin, L.A. Visualization of Particle Complexes in the Plasma Membrane of Micrasterias Denticulata Associated with the Formation of Cellulose Fibrils in Primary and Secondary Cell Walls. J. Cell Biol. 1980, 84, 327–339.
- Doblin, M.S.; Kurek, I.; Jacob-Wilk, D.; Delmer, D.P. Cellulose Biosynthesis in Plants: From Genes to Rosettes. Plant Cell Physiol. 2002, 43, 1407–1420.
- Saxena, I.M.; Brown, R.M. Cellulose Biosynthesis: Current Views and Evolving Concepts. Ann. Bot. 2005, 96, 9–21.
- Haigler, C.H.; Brown, R.M.; Benziman, M. Calcofluor White ST Alters the in Vivo Assembly of Cellulose Microfibrils. Science 1980, 210, 903–906.
- Cousins, S.K.; Brown, R.M. Cellulose I Microfibril Assembly: Computational Molecular Mechanics Energy Analysis Favours Bonding by van Der Waals Forces as the Initial Step in Crystallization. Polymer 1995, 36, 3885–3888.
- Cousins, S.K.; Brown, R.M. X-Ray Diffraction and Ultrastructural Analyses of Dye-Altered Celluloses Support van Der Waals Forces as the Initial Step in Cellulose Crystallization. Polymer 1997, 38, 897–902.
- Cousins, S.K.; Brown, R.M. Photoisomerization of a Dye-Altered β-1,4 Glucan Sheet Induces the Crystallization of a Cellulose-Composite. Polymer 1997, 38, 903–912.
- McCorsley, C.C. Process for Shaped Cellulose Article Prepared from a Solution Containing Cellulose Dissolved in a Tertiary Amine N-Oxide Solvent. U.S. Patent nº 4246221, 20 January 1981.
- Medronho, B.; Lindman, B. Competing Forces during Cellulose Dissolution: From Solvents to Mechanisms. Curr. Opin. Colloid Interface Sci. 2014, 19, 32–40.
- Medronho, B.; Lindman, B. Brief Overview on Cellulose Dissolution/Regeneration Interactions and Mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508.
- Budtova, T.; Navard, P. Cellulose in NaOH–Water Based Solvents: A Review. Cellulose 2016, 23, 5–55.
- Nägeli, C. Über Den Inneren Bau Der Vegetabilischen Zellmembranen. Sitzber Bay Akad. Wiss. München 1984, 1, 282–323.
- Pennetier, G. Note Micrographique Sur Les Altérations Du Cotton. Bull. Soc. Ind. Rouen. 1883, 11, 235–237.
- Fleming, N.; Thaysen, A.C. On the Deterioration of Cotton on Wet Storage. Biochem. J. 1921, 15, 407–414.1.
- Fleming, N.; Thaysen, A.C. On the Deterioration of Cotton on Wet Storage. Biochem. J. 1920, 14, 25–28.1.
- Tripp, V.W.; Rollins, M.L. Morphology and Chemical Composition of Certain Components of Cotton Fiber Cell Wall. Anal. Chem. 1952, 24, 1721–1728.
- Hock, C.W. Microscopic structure. In Cellulose and Cellulose Derivatives (Part 1); Ott, E., Spurlin, H.M., Grafflin, M.W., Eds.; Interscience: New York, NY, USA, 1954; pp. 347–392.
- Warwicker, J.O.; Jeffries, R.; Colbran, R.L.; Robinson, R.N. A review of the literature on the effect of caustic soda and other swelling agents on the fine structure of cotton. In Pamphlet 93 Shirley Inst.; Cotton, Silk and Man-made Fibres Research Association: Manchester, UK, 1966; p. 247.
- Chanzy, H.; Noe, P.; Paillet, M.; Smith, P. Swelling and Dissolution of Cellulose in Amineoxide/Water System. J. Appl. Polym. Sci. 1983, 37, 239–259.
- Cuissinat, C.; Navard, P. Swelling and Dissolution of Cellulose Part 1: Free Floating Cotton and Wood Fibres in N-Methylmorpholine-N-Oxide–Water Mixtures. Macromol. Symp. 2006, 244, 1–18.
- Neale, S.M. 30—The Swelling of Cellulose, and its Affinity Relations With Aqueous Solutions. Part I—Experiments on the Behaviour of Cotton Cellulose and Regenerated Cellulose in Sodium Hydroxide Solution, and their Theoretical Interpretation. J. Text. Inst. Trans. 1929, 20, T373–T400.
- Lindström, T.; Carlsson, G. The Effect of Carboxyl Groups and Their Ionic Form during Drying on the Hornification of Cellulose Fibers . Sven. Papperstidning 1983, 85, r146–r151.
- Scallan, A.M. The Effect of Acidic Groups on the Swelling of Pulps: A Review. Tappi J. 1983, 66, 73–75.
- Katz, S.; Liebergott, N.; Scallan, A.M. A Mechanism for the Alkali Strengthening of Mechanical Pulps. TAPPI J. Tech. Assoc. Pulp Pap. Ind. 1981, 64, 97–100.
- Katz, S.; Scallan, A.M. Ozone and Caustic Soda Treatments of Mechanical Pulp. Tappi J. 1983, 66, 85–87.
- Zhang, S.; Wang, W.; Li, F.; Yu, J. Swelling and dissolution of cellulose in NaOH aqueous solvent systems. Cell. Chem. Technol. 2013, 47, 671–679.
- Ehrhardt, A.; Groner, S.; Bechtold, T. Swelling Behavior of Cellulosic Fibers—Part I: Changes in Physical Properties. Fibres Text. East. Eur. 2007, 15, 46–48.
- Mahmud-Ali, A.; Bechtold, T. Aqueous Thiocyanate–Urea Solution as a Powerful Non-Alkaline Swelling Agent for Cellulose Fibres. Carbohydr. Polym. 2015, 116, 124–130.
- Forest Products Laboratory (U.S.). Forest Products Laboratory Urea-Plasticized Wood (Uralloy); U.S. Dept. Laboratory, Forest Service, Forest Products Laboratory, Madison, Wisconsin in Cooperation with the University of Wisconsin Forest Products: Madison, WI, USA, 1943.
- Brown, W.F. Vulcanized Fibre—An Old Material with a New Relevancy. In Proceedings of the Electrical Insulation Conference and Electrical Manufacturing and Coil Winding Conference, Cincinnati, OH, USA, 28 October 1999.
- Halonen, H. Structural Changes during Cellulose Composite Processing. Ph.D. Thesis, KTH, Stockholm, Sweden, 2012.
- Künne, B.; Dumke, D. Vulcanized Fiber as a High–Strength Construction Material for Highly Loaded Construction Units. In Proceedings of the International Paper Physics Conference & 8th International Paper and Coating Chemistry, Stockholm, Sweden, 10–14 June 2012.
- Piltonen, P.; Hildebrandt, N.C.; Westerlind, B.; Valkama, J.-P.; Tervahartiala, T.; Illikainen, M. Green and Efficient Method for Preparing All-Cellulose Composites with NaOH/Urea Solvent. Compos. Sci. Technol. 2016, 135, 153–158.
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836.
- Soykeabkaew, N.; Arimoto, N.; Nishino, T.; Peijs, T. All-Cellulose Composites by Surface Selective Dissolution of Aligned Ligno-Cellulosic Fibres. Compos. Sci. Technol. 2008, 68, 2201–2207.
- Nishino, T.; Arimoto, N. All-Cellulose Composite Prepared by Selective Dissolving of Fiber Surface. Biomacromolecules 2007, 8, 2712–2716.
- Sjöström, E. Wood Chemistry, Fundamentals and Applications, 2nd ed.; Press, A., Ed.; Elsevier: San Diego, CA, USA, 1993; ISBN 9780080925899.
- Eklund, D.; Lindström, T. Paper Chemistry: An Introduction, 1st ed.; DT Paper Science Publications: Grankulla, Finland, 1991.
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; Furó, I.; Berglund, L.A.; Wohlert, J. Cellulose and the Role of Hydrogen Bonds: Not in Charge of Everything. Cellulose 2022, 29, 1–23.
- Bodvik, R.; Dedinaite, A.; Karlson, L.; Bergström, M.; Bäverbäck, P.; Pedersen, J.S.; Edwards, K.; Karlsson, G.; Varga, I.; Claesson, P.M. Aggregation and Network Formation of Aqueous Methylcellulose and Hydroxypropylmethylcellulose Solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 354, 162–171.
- Zhang, L.; Ruan, D.; Gao, S. Dissolution and Regeneration of Cellulose in NaOH/Thiourea Aqueous Solution. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1521–1529.
- Isogai, A.; Atalla, R.H. Dissolution of Cellulose in Aqueous NaOH Solutions. Cellulose 1998, 5, 309–319.
- Cao, N.-J.; Xu, Q.; Chen, C.-S.; Gong, C.S.; Chen, L.F. Cellulose Hydrolysis Using Zinc Chloride as a Solvent and Catalyst. Appl. Biochem. Biotechnol. 1994, 45–46, 521–530.
- Åstrand, P.-O.; Karlström, G.; Engdahl, A.; Nelander, B. Novel Model for Calculating the Intermolecular Part of the Infrared Spectrum for Molecular Complexes. J. Chem. Phys. 1995, 102, 3534–3554.
- Shinoda, K. “Iceberg” Formation and Solubility. J. Phys. Chem. 1977, 81, 1300–1302.
- Kihlman, M.; Medronho, B.F.; Romano, A.L.; Germgard, U.; Lindman, B. Cellulose Dissolution in an Alkali Based Solvent: Influence of Additives and Pretreatments. J. Braz. Chem. Soc. 2013, 24, 295–303.
- Zhou, J.; Zhang, L. Solubility of Cellulose in NaOH/Urea Aqueous Solution. Polym. J. 2000, 32, 866–870.
- Cai, J.; Zhang, L. Rapid Dissolution of Cellulose in LiOH/Urea and NaOH/Urea Aqueous Solutions. Macromol. Biosci. 2005, 5, 539–548.
- Kronberg, B. The Hydrophobic Effect. Curr. Opin. Colloid Interface Sci. 2016, 22, 14–22.
- Biermann, O.; Hädicke, E.; Koltzenburg, S.; Müller-Plathe, F. Hydrophilicity and Lipophilicity of Cellulose Crystal Surfaces. Angew. Chemie Int. Ed. 2001, 40, 3822–3825.
- Yamane, C.; Aoyagi, T.; Ago, M.; Sato, K.; Okajima, K.; Takahashi, T. Two Different Surface Properties of Regenerated Cellulose Due to Structural Anisotropy. Polym. J. 2006, 38, 819–826.
- Miyamoto, H.; Umemura, M.; Aoyagi, T.; Yamane, C.; Ueda, K.; Takahashi, K. Structural Reorganization of Molecular Sheets Derived from Cellulose II by Molecular Dynamics Simulations. Carbohydr. Res. 2009, 344, 1085–1094.
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046.
- Haigler, C.H.; White, A.R.; Brown, R.M.; Cooper, K.M. Alteration of in Vivo Cellulose Ribbon Assembly by Carboxymethylcellulose and Other Cellulose Derivatives. J. Cell Biol. 1982, 94, 64–69.
More
