1. Introduction
The application of alkali-activated binders (AABs) was introduced due to the lack of ordinary Portland cement (OPC)OPC availability in the post-World-War-II period [1][3]. Since the late 1990s, the usage of AABs has been investigated as a sustainable solution in the construction industry [2][4]. In the initial investigations of AABs’ environmental impacts, Davidovits stated that 1 tonne of geopolymer cement generates 0.18 tonnes of CO2, corresponding to an about five to six times lower value than OPC [3][5]. In comparison, Duxson et al. emphasised that the use of geopolymer binders can result in an 80% or even greater decrease in CO2 emissions compared to OPC [4][6].
In general, selecting the suitable AA depends on the precursor’s composition
[1][3]. Two modes of AA production have been developed:
-
One-part mixtures combine dry alkali powder, solid aluminosilicate raw material, and water. They are suitable for in situ applications due to their advantageous handling characteristics compared to two-part mixtures containing a viscous alkali solution. Since the usage and packaging of this type are similar to those of cement, its utilisation seems promising.
-
Two-part mixtures are formed by combining an aqueous alkali solution, solid aluminosilicate raw material, and water. They have been recognised as suitable for precast applications. However, they pose a disadvantage, as they require the handling of large amounts of corrosive, hazardous, and viscous alkali solutions, which has led to the development of one-part mixtures
[5][8].
However, precursors can be naturally occurring or industrial and agricultural by-products and/or waste. They are mainly composed of SiO2, Al2O3, and CaO [6][9]. By-products from the energy and mining industries have received much attention with respect to their use as a potential feedstock for AABs [7][10]. Moreover, solid waste generated throughout mining activities has been recognised as causing severe environmental pollution, and its utilization as raw material would be highly beneficial [8][11]. The producers of these by-products can benefit from reductions in their required storage and rehabilitation costs if the by-product is disposed of as waste. Additionally, from their sale as raw materials, some economic profit is also possible [7][10]. Various types of agricultural waste have also been investigated as raw materials for producing AABs [6][9]. On the other hand, bulk chemicals (e.g., sodium hydroxide, sodium silicate, etc.) are commonly utilised as AAs, while there have been few investigations on the use of industrial and agricultural by-products and/or wastes as alternative alkali sources [1][3]. In general, alkali hydroxide (ROH), non-silicate salts of weak acids (R2CO3, R2S, and RF), and silicic salts of R2O·(n)SiO2 are widely used, where R corresponds to an alkali metal, i.e., Na, K, or Li [2][4].
2. Obstacles Related to Technical Performance
The factors influencing the synthesis of AABs are the composition, fineness, quantity, and source of the precursor material; the type and composition of the AA employed; the quantity of free water used; and the type and duration of curing. These aspects affect both the fresh and hardened properties of AABs as well as their long-term performance. Various studies have investigated the effect of the chemical composition of AABs’ ingredients on their material properties. For instance, the mechanical properties of metakaolin (MK)-based geopolymers have been investigated with respect to the effect of Si/Al, Al/M (M is an alkali cation), and H
2O/Na
2O. In a study conducted by Lahoti et al., the Si/Al ratio was the most significant factor influencing compressive strength, followed by the Al/Na ratio
[9][139]. On the other hand, the calcium content of ground-granulated-blast-furnace-slag (GGBFS)-based AABs seems to influence its mechanical properties, wherein excessive calcium content can confer an adverse effect
[10][140]. Furthermore, control over the fresh properties is difficult, which is especially important because of the scarce availability of suitable admixtures. Notably, an excessive amount of water present in the mix of AABs decreases their strength because it is not essential in chemical reactions, unlike cement hydration
[6][9]. It is mostly physically bonded and tends to increase the porosity of alkali-activated material (AAM). It may also negatively influence mechanical and durability properties
[11][141].
One of the challenges in the broader application of AABs is the curing method employed. AABs based on glassy aluminosilicates (e.g., fly ash) typically require elevated curing temperatures for stable hydrate reaction and to gain strength
[2][4]. Heat curing is performed in a moderate temperature range from 20 to 80 °C
[12][142]. However, the advantageous material property of AABs is their thermal stability, which can be beneficial when the construction materials used must be thermally stable
[13][133]. Additional issues are based on a scarcity of data, an incomplete understanding of the mix’s design, and limited information on long-term performance. Finally, the scarcity of the standards, specifications, and regulations for the production and application of AABs also hinders their broader utilisation
[6][9].
2.1. Precursors
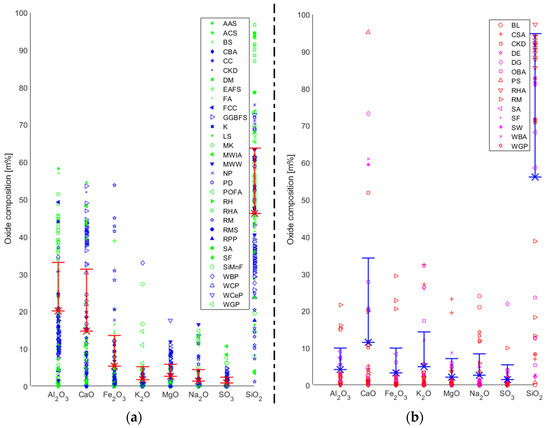
As mentioned above, one of the largest concerns in this field is insufficient uniformity in the composition of the precursor materials and hence its impact on the resulting AABs
[6][9]. It has been recognised that AABs are highly sensitive to the chemical composition of the precursor, which varies based on the source material (
Figure 1a). One of the most important factors for producing a stable AAB is the use of highly amorphous precursors possessing adequate reactive glassy content. Additionally, its water demand should be relatively low, and it should release easily
[10][140].
Figure 1.
Chemical composition of (
a
) precursors and (
b
) alternative alkali activators.
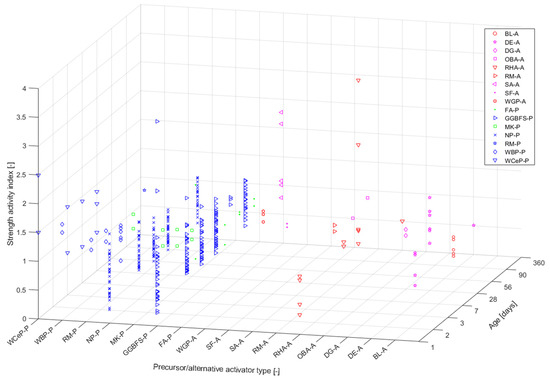
Figure 2 shows the strength ratio results representing a ratio of the compressive strength of the generated AAM and OPC-based material with respect to the specific precursor type used (i.e., cement paste, mortar, or concrete). The colour- and symbol-coding follows the scheme used in
Figure 1a for the respective precursors. Each precursor type was assigned a code, X-P, where X corresponds to an abbreviation of the respective raw material and P stands for precursor (another code, X-A, will be discussed in the next section). The data presented in
Figure 2 demonstrate results obtained for mortar
[14][15][28,81], concrete
[16][17][55,69], and paste specimens
[18][130]. Only papers referencing an OPC-based material are presented. For the most part, a combination of sodium silicate (SS) and sodium hydroxide (SH) was used as an activator, with varied concentrations and combinations (with some exceptions regarding the use of alternative AAs
[14][28]).
Figure 2.
Strength ratio results for alkali-activated materials.
The reactivity of the precursors used to date cannot be simply based on their broad typologies. Silica and alumina from the source materials are among the most influential factors with respect to the material properties of AAB. Another oxide that significantly contributes to the reaction kinetics of AAB materials is that formed with calcium. It has been demonstrated that the workability of AAMs is reduced when a low-calcium precursor (e.g., fly ash) is partially/fully replaced by a high-calcium precursor (e.g., GGBFS). In general, the increase in the amount of calcium increases the rate of hydration (which is the opposite chemical process to polymerisation) while also consuming water. Due to these two effects, the viscosity can be increased, while the setting time can be decreased of the resulting AAM.
The reactivity of the precursors used to date cannot be simply based on their broad typologies. Silica and alumina from the source materials are among the most influential factors with respect to the material properties of AAB. Another oxide that significantly contributes to the reaction kinetics of AAB materials is that formed with calcium. It has been demonstrated that the workability of AAMs is reduced when a low-calcium precursor (e.g., fly ash) is partially/fully replaced by a high-calcium precursor (e.g., GGBFS). In general, the increase in the amount of calcium increases the rate of hydration (which is the opposite chemical process to polymerisation) while also consuming water. Due to these two effects, the viscosity can be increased, while the setting time can be decreased of the resulting AAM.
Moreover, heat curing is usually applied to low-calcium systems because of the slow polymerisation process, which is also one of the major disadvantages of utilising this kind of AAC cast in situ. However, when GGBFS is considered, air-dry curing can be applied [2][4].
2.2. Alkali Activators
The AAs suitable for AABs could be any sufficiently water-soluble compound whose use results in a high pH and that is able to provide alkali/alkali earth cations (e.g., alkali-hydroxides/sulfates/carbonates/silicates)
[1][3]. The adverse effects of the high quantity of AAs on the cost of production, strength gain, and performance in aggressive environmental conditions has been acknowledged
[6][9]. Some of the most used AAs are SH and SS. Generally, SS results in a more compact and denser material with higher mechanical strength than SH. It also offers greater advantages regarding handling and usage compared to aqueous activators
[5][8]. In AABs’ systems, alkali hydroxides (e.g., SH) provide OH
−, and, based on the interdiffusion and ion exchange of solutes and aluminosilicate network modifier cations (e.g., Ca
2+) of the precursors, the dissolution of the precursor is promoted. The dissolution of most of the precursors increases as a function of an increasing alkali hydroxide concentration. However, a considerable concentration of the alkali hydroxide in AABs can result in efflorescence problems. On the other hand, when silicate-based activators are used, the precursors’ activation mechanism is similar to hydroxide-based activators with additional soluble silicate available. Moreover, carbonates, as activators, could aid CO
2 binding in the presence of calcium in the system. Adding alkali sulphates as activators can be a viable solution for durability enhancement, wherein chloride binding properties can be introduced. Nevertheless, alkali carbonates and sulphates are unsuitable for low-calcium precursors (e.g., MK) due to the relatively low pH value introduced in the mix. In comparison, alkali sulphates result in a substantially lower heat of hydration than most other activators, which could be beneficial for application to AABs. Finally, aluminate-based activators constitute another type of AA, which offer the benefit of dissolved alumina
[1][3].
However, dual AAs of SH and SS have been recognised as highly efficient for use in AABs containing both low and high calcium content. In such a system, SH is the main activator providing hydroxide anions (OH−), whereas SS acts as an auxiliary activator providing soluble silica. Moreover, the effect of AAs on the final and initial setting times has been recognised, where at a higher dosage of SS, the increased SS/SH ratio causes an accelerated rate of dissolution of the precursor, thereby altering reaction kinetics and the condensation process. This leads to fast initial and final setting times. However, the SS/SH ratio or the concentration of SH causing an increase in the overall liquid content to binder ratio substantially delays setting.
Since environmental issues are a significant concern in the industry and there are widely available industrial and agricultural waste streams with no market value, alternative solutions to widely used primary AAs have been extensively investigated. Such approaches are especially viable for AA because, for example, silica can be extracted from glass waste such as RHA, silica fume (SF), and many other biomass ashes and combined with alkali hydroxides through hydrothermal or thermochemical methods, resulting in the production of SS
[1][3].
In Figure 1b, the chemical compositions of various types of industrial and agricultural waste used to generate AAs are presented.
3. Obstacles Related to Environmental Performance
Although studies identifying AABs as constituting a sustainable alternative to OPC have been published since the beginning of this century, studies that systematically approach the environmental performance of AAMs are still scarce. If only greenhouse (GHG) emissions (i.e., global warming potential—GWP) are evaluated in environmental impact assessments, the production of AAMs could lead to lower values than those contributed by conventional materials. However, other than GWP, various environmental impact categories should be assessed, such as ozone layer depletion (ODP), acidification potential (AP), human toxicity (HTP), etc. Even in one of the first studies tackling the environmental performance of AABs, Davidovits not only touched upon CO
2 emissions but also the heavy metal waste encapsulation possibilities of geopolymer cement as an environmentally driven application
[3][5]. The results could be surprising if a comprehensive evaluation is performed, wherein it can be observed that AAMs are projected to be less sustainable than widely anticipated
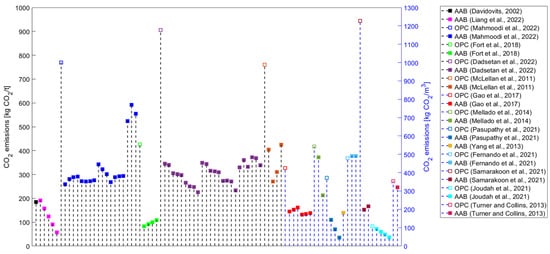
[1][3]. Most studies have focused on estimating AAMs’ GHG emissions (
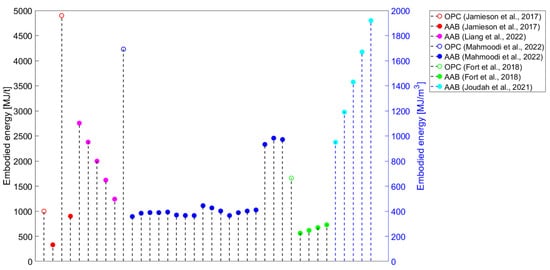
Figure 3) or embodied energy (
Figure 4). Even if only the evaluation of these two environmental impact categories is considered, a comparison of the results of the studies is complex because there are discrepancies in the methods used.
Figure 3.
CO
emissions results for alkali-activated materials from the literature [4,5,10,46,48,59,60,67,101,102,127,130,144,145,146].
Figure 4. Embodied energy results for alkali-activated materials from the literature [18][19][20][28][30]. Embodied energy results for alkali-activated materials from the literature [19,46,48,130,145].
Some of the most critical steps in LCA are defining functional units and system boundaries. A functional unit is defined as the quantified performance of a product system for use as a reference unit. At the same time, a system boundary is a set of criteria specifying the unit processes forming part of a product system
[31][12]. The definition of these two steps is essential for an LCA of a construction material because when compared to other solutions, care should be taken as to how this comparison would be conducted. For example, the material with the lowest environmental impact per unit volume or mass is not always the one with the lowest environmental impact when used in a structure throughout its service life
[32][149]. Based on the data gathered
(Table 3), functional units were mainly defined based on mass (i.e., t) or volume (i.e., m
3). On the other hand, system boundaries differed significantly. Some of the studies considered the cradle-to-gate approach, which would include raw material production and the mixing of the constituents.
3.1. Precursors
When OPC concrete was compared with AAC using GGBFS and FA, the obtained levels of carbon emissions were two times lower
[21][59]. The use of MK as a precursor was compared to secondary raw materials (i.e., FA and GGBFS), which were associated with AABs’ more favourable environmental impacts
[12][142]. Ameri et al. showed that using various types of precursors, namely, GGBFS, MK, waste ceramic powder (WCeP), and WBP, for AAM could lead to an up to 50% reduction in CO
2 emissions and a 25% decrease in energy consumption compared to cement mortar
[15][81]. Compared to merely the binary precursor of GGBFS and FA, the ternary precursor of GGBFS, FA, and WBP reduced GHG emissions and energy consumption by roughly 70% and 55%, respectively.
[19][46].
Moreover, in a study that fully utilised construction-and-demolition-sourced waste as WBP, WCeP, and waste concrete powder (WCP) in a ternary precursor, a decrease of about 86% in embodied energy and 76% in carbon dioxide compared to an OPC binder was shown
[20][48]. Even the utilisation of solely WBP as a precursor resulted in a reduction of consumed energy by 63
[18][130]–85%
[33][47] and GHG emissions by 78
[33][47]–81%
[18][130] for a mixture of alkali-activated paste with the best mechanical properties compared to a cement one. Foam concrete investigation using WBP as a precursor has shown that GHG emissions can be reduced by up to 88%. However, it was emphasised that when choosing a suitable product, one should also consider the intended application with respect to the required mechanical and thermal properties
[26][127].
3.2. Alkali Activators
One of the first LCAs of the AABs was performed by Weil et al. in 2009. They emphasised that the application of SS and SH should be reduced or replaced by more eco-friendly activators
[12][142]. Around 60 Mt of SH is produced annually, while the amount of SS is around 1/10th of this value
[34][150]. SS has been identified as one of the most significant contributors to the environmental impacts of AAMs, which is mainly due to the high level of energy consumption related to its production
[5][8]. In the literature, conventional AAs were recognised as having a significant impact on AAMs’ GHG emissions
[2][7][14][15][18][24][25][26][27][33][35][4,10,28,47,81,101,102,127,130,144,147]. It is estimated that 1.1 kg and 1.2 kg of CO
2 are emitted per 1 kg of SH and SS production, respectively
[1][3].
Compared to OPC, the values for GHG emissions were lower in the MK-based geopolymer considering cradle-to-gate LCA. However, the opposite was noted with respect to the energy demand. The energy demand results were highly affected by the 3000 MJ of electricity needed to produce one ton of SS
[36][85].
While using industrial residue as an AA may not decrease the total GWP of AAMs, it might result in lower toxicity (in terms of terrestrial, freshwater, and marine environments) and less ozone depletion. So, the least processed raw materials produce the best outcomes across all categories
[14][28]. As opposed to SS solutions, the RHA-derived solution has been proven to improve the environmental sustainability of AABs
[37][114]. AAs derived from SH solutions and two different soluble silicate solutions, namely, commercial SS and chemically modified RHA, were investigated by Passuello et al.
[38][118]. It was demonstrated that the selection of the activator impacted many categories evaluated in the LCA of the pastes. On the other hand, AABs produced using RHA resulted in lower impacts than other evaluated geopolymer pastes for all the categories considered
[38][118].
Moreover, generating a WGP-based alternative AA resulted in 18% lower CO
2 emissions than the generation of SS
[24][101]. Abdulkareem et al.
[5][8] performed a comparative LCA in which AAMs produced using chemically modified one- and two-part waste-derived activators were compared to conventionally used ones. For this purpose, they used WGP and RHA. In the findings, it was determined that the AAMs with WGP and RHA resulted in 62%, 61%, 56%, and 76% lower values in the four investigated impact categories (climate change, fossil depletion, photochemical ozone creation, and terrestrial acidification) compared to the conventional activators
[39][2].
3.3. Regional Context
Mellado et al.
[23][67] investigated AAMs based on data for Spain, and one section of the study examined emissions associated with the components of the binder, excluding mixing and curing but including the external grinding of fluid catalytic cracking catalyst (FCC) and RHA as well as the transportation of the materials from their point of origin. The contribution of transportation for OPC was 2%, while for AAMs this value was equal to 14% when the binder was made with FCC, commercial waterglass, SH, and water. In the case of replacing commercial waterglass with RHA, the contribution of transportation was 15%. This was also mainly due to the more considerable distances associated with the raw materials for AAMs
[23][67].
4. Obstacles Related to Economic Performance
4.1. Precursors
The contribution of the precursor type used to an AAM’s cost is based on its production cost, the distance for which it must be transported, and the type of curing method employed (i.e., heat/ambient curing). Samarakoon et al. found that when WGP was used as both the partial activator and precursor, a 15% decrease in cost compared to an OPC binder was achieved
[29][146]. Joudah et al.
[28][145] investigated the production cost of AAMs made with FA, GGBFS, and WCeP. About fivefold higher values were obtained for WCeP compared to FA in terms of the cost for preparation as a raw material. The level of energy consumption for producing GGBFS was significantly higher than WCeP and FA. Additionally, GGBFS was associated with the highest transportation distance from all the precursor ingredients.
4.2. Alkali Activators
In most of the studies concerning the LCC of AAMs, the type of AA used has been recognised as one of the most influential factors
[5][14][22][29][36][37][8,28,60,85,114,146]. Regarding the cradle-to-gate LCC of MK-based geopolymers, its cost was higher than that of OPC. SS was identified as one of the main contributors
[36][85]. Moreover, in a study by Fernando et al., a 48% increment was observed for AACs compared to OPC concrete, which was mainly affected by the high cost of AAs. AAs accounted for around 74% of the total cost of AAC
[22][60]. On the other hand, in the studies that focused on alternative activators, a decrease in the total cost of AABs was visible
[5][14][29][37][8,28,114,146].
4.3. Regional Context
The transportation costs for the raw materials used in AABs are especially important for relatively isolated regions such as Australia. McLellan et al.
[7][10] examined the LCC of OPC and geopolymer pastes based on Australian practices. The assessment covered the life-cycle stages up to the production of the binder. It was recognised that the critical factors influencing the costs of geopolymers are the source location, the energy source, and the transportation mode. When only production was considered, geopolymers were acknowledged as potentially competitive in terms of cost. However, when transportation was included in the assessment, a negative impact was observed due to long distances.