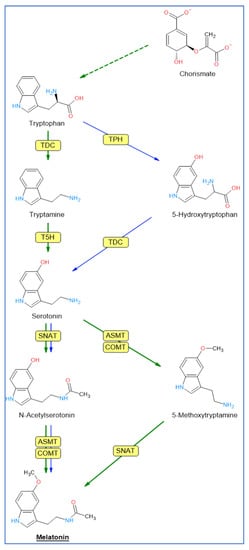
2. Biosynthetic Pathway of Melatonin
Tryptophan, an amino acid that is synthesized from chorismic acid in plants, is the origin of the melatonin biosynthesis pathway
[4][43][44][4,72,73] in animal and plant cells, but differs in some steps. In animal cells, tryptophan is converted to 5-hydroxytryptophan by tryptophan hydroxylase (TPH), an enzyme that apparently has not been identified in plants
[45][46][74,75].
In plants, tryptophan is converted to tryptamine by the enzyme tryptophan decarboxylase (TDC) (
Figure 1). Tryptamine is then converted into 5-hydroxytryptamine (serotonin) by tryptamine 5-hydroxylase (T5H), an enzyme that has been extensively studied in rice, and which could act on many substrates. Serotonin was
N-acetylated by serotonin
N-acetyltransferase (SNAT).
N-acetylserotonin is then methylated by acetylserotonin methyl transferase (ASMT), a hydroxyindole-
O-methyltransferase that generates melatonin. In plants, methylation of
N-acetylserotonin can also be performed by a caffeic acid
O-methyltransferase (COMT), a class of enzyme that can act on a variety of substrates, including caffeic acid and quercetin
[47][76]. In plants, serotonin may also be transformed into 5-methoxytryptamine by ASMT/COMT to generate melatonin after the action of SNAT
[48][77]. This route would occur in senescence and/or stress situations
[46][49][75,78].
Figure 1 shows the steps of the biosynthesis of melatonin in animals (mainly mammals) and plants.
Figure 1. Pathways of melatonin biosynthesis in plants (green arrows) and animals (blue arrows). Enzyme abbreviations: TDC = tryptophan decarboxylase; TPH = tryptophan hydroxylase; T5H = tryptamine 5-hydroxylase; SNAT = serotonin N-acetyltransferase; ASMT = acetylserotonin methyl transferase; COMT = caffeic acid O-methyltransferase.
3. Physiological Roles of Phytomelatonin
The functions of phytomelatonin in plants have been widely studied, observing that phytomelatonin is a protective biomolecule that activates tolerance and resistance responses in plants, playing an important role against biotic (bacteria, viruses, fungi, insects, parasitic nematodes and weeds) and abiotic stressors (salinity, drought, waterlogging, UV-radiation, heavy metal, heat, cold, mineral deficit/excess, and pesticides)
[50][51][52][45,47,58].
Melatonin/phytomelatonin plays an important role in plants as a large antioxidant molecule, similar to how it occurs in animal cells, generally acting as an excellent free radical scavenger, specifically on ROS and RNS
[26][28][53][26,28,79]. The direct antioxidant activity of melatonin that neutralizes several ROS/RNS and other free radical species that can damage cells was demonstrated in several in vitro and in vivo models
[54][55][56][57][80,81,82,83]. It also activates the antioxidant redox response, upregulating various transcription factors that trigger the expression of antioxidant enzymes such as superoxide dismutases, catalases, peroxidases, and those involved in the ascorbate-glutathione cycle, among others
[28][44][58][28,73,84].
Phytomelatonin is considered a plant master regulator because it regulates the levels and actions of plant hormones. The endogenous levels of auxin, gibberellins (GA), cytokinins (CK), abscisic acid (ABA), ethylene (ET) and other phytohormones such as brassinosteroids, jasmonates (JA) and salicylates (SA) are affected by the action of phytomelatonin through up- or downregulation of transcripts of some biosynthesis/catabolism enzymes, and also hormone-related regulatory factors
[51][59][60][47,57,85].
On the other hand, it should be noted that photosynthesis, photorespiration, and stomatal regulation, which are key pieces of the water and carbon economy in plants, are strongly regulated by phytomelatonin. Moreover, the metabolic pathways of carbohydrates, lipids and nitrogen and sulfur compounds are modulated by phytomelatonin, including the osmoregulatory response in stressful situations. In secondary metabolism, phytomelatonin induces the biosynthesis of flavonoids, anthocyanins and carotenoids, among other molecules
[61][62][63][44,51,62].
Phytomelatonin also promotes the rooting process for primary and adventitious roots
[64][86], and it regulates leaf senescence, delaying it
[65][87]. In fruit post-harvest, phytomelatonin regulates ethylene and lycopene content, as well as cell wall-related enzymes and primary and secondary metabolism. It also helps preserve cut flowers, delaying senescence, and induces parthenocarpy in fruiting
[66][48].
Due to the ability of phytomelatonin to protect plants against stresses, this molecule could be used as a safener in crops. This agronomical application consists of using phytomelatonin in combination with pesticides, not only to increase the plant tolerance to the possible stress caused by the pesticide (abiotic stress) but also to increase the pesticide efficacy against the biotic stressor
[67][68][69][70][53,88,89,90]. Another application of the effect of phytomelatonin under abiotic stresses is its use postharvest. Phytomelatonin reduces the effect of cold storage of vegetables and fruits by minimizing the damage caused by ROS, improving the quality and commercial shelf-life of fruits and vegetables
[71][72][73][74][91,92,93,94].
Lastly, its role in bacterial, fungal, and viral pathogenic infections should be emphasized. Phytomelatonin slows damage and stimulates systemic acquired resistance (SAR), which contributes to increasing both crop health and postharvest health quality
[66][73][75][76][77][48,52,61,93,95].
4. Melatonin in Plant Disease Biocontrol
There are different approaches that can be used to prevent, mitigate, or control plant diseases. One of them is the application of chemical pesticides, which are used both for preventive as well as for curative disease management
[78][79][96,97]. However, there is a concern about the negative effects of chemical pesticides due their possible harmful effects on human health, the environment, as well as their effect on the promotion of new resistant pathogens
[80][81][82][98,99,100].
There is increasingly stricter legislation in relation to the accessibility and use of efficient pesticides and, therefore, their use is currently declining
[83][101]. Consequently, one study has focused its efforts on developing alternatives to synthetic chemicals for pests and diseases control, some of these being alternatives to so-called biological control.
The term biocontrol refers to the use of naturally occurring (micro-)organisms to control plant diseases or pests
[84][102]. The organism that suppresses the pest or pathogen is found widely in nature, including bacteria, fungi, viruses, yeasts, and protozoans. It can control plant diseases directly, which can be achieved through parasitism, antibiosis, or competition for nutrients or infection sites, or indirectly, where the biocontrol organism induces plant-mediated responses allowing the plant to react faster and more efficiently in case of subsequent pathogen attack
[85][103].
The induced resistance strategy in plants can be indirectly carried out, not only through an organism but also using elicitors; that is, a natural molecule that mimics a pathogen attack or a state of danger, also by living organisms. Plant-induced resistance may represent an interesting strategy for crops
[86][104].
Considering this, melatonin can be an excellent candidate to be used in biocontrol treatments as elicitor molecule. There are currently several studies conducted on the effect of melatonin treatment in the control or reduction of infectious diseases in plants, such as those caused by fungi, bacteria, and viruses.
Plant viruses produce local lesions and also cause systemic damage, resulting in malformations, stunting, and chlorosis in plant tissues, even if their hosts are often biotrophic pathogens
[77][87][68,95]. Viral diseases in plants cause severe economic losses due to agricultural production and have hindered sustainable agricultural development globally for a long time. Unlike diseases induced by fungi and bacteria, viral infections are more difficult to control once the plants are infected
[88][89][144,145].
In recent decades, various strategies have been developed to control viral diseases, including the breeding of virus-resistant/tolerant cultivars by conventional breeding techniques
[90][146] and the use of eco-friendly chemical compounds that induce systemic resistance
[91][143].
Chemical priming may be considered a timely and successful management technique to induce resistance/tolerance to viruses of plants. Several eco-friendly compounds that are considered non-toxic, biodegradable, and also biocompatible oligomers, such as proteins, polysaccharides and small molecules (alkaloids, flavonoids, phenolics, essential oils) from plants, proteins and polysaccharides from microorganisms, polysaccharides from algae and oligochitosan from animals, can be used to induce plant resistance to viruses
[92][93][147,148].
Concerning virus infection in plants, there are a few reports (since the first studies were conducted in 2019) based on the study of the interaction of plant viruses with melatonin and its possible role as an inducer of viral resistance in plants. One of these studies was conducted by Chen and cols. (2019) on the potential role of melatonin in the eradication of
Apple Stem Grooving Virus (ASGV) infection in in vitro Gala apple cultivars
[94][141]. Apple is generally propagated vegetatively by grafting, resulting in transmission of the virus from generation to generation; therefore, it is important to obtain virus-free apple planting materials. Apple shoot segments excised from ASVG virus-infected shoots were cultured in various media supplemented with 0, 10, 15, or 20 µM melatonin and were maintained at 22 °C under a 16 h photoperiod. Ten samples were included in each treatment of three replicates. Treatments of 15 µM melatonin were the most efficient in promoting the number and length of the shoots, as well as the high level of endogenous hormone indole-3-acetic acid. On the other hand, in vitro culture of the virus-infected shoots tips in the medium with 15 µM melatonin resulted in 95% of these shoots being virus-free, while no virus-free shoots were obtained in shoot tips of the virus infected shoots cultured without melatonin. In addition, those plants that continued to be infected, even with 15 µM melatonin in the medium, showed a lower viral load than infected plants grown without melatonin. The virus localization showed that exogenous application of melatonin enlarged the virus-free area of the virus-infected shoot tips. The authors concluded that the exogenous application of melatonin can efficiently eradicate ASGV, being the frequency of the virus eradication related to the melatonin concentration used and the culture time duration on melatonin-containing shoot proliferation medium. Inclusion of 15 µM melatonin in the medium to proliferate shoots for 4 weeks followed by shoot tip culture was found to efficiently eradicate ASGV. This procedure produced 100% of survival and 85% of shoot regrowth levels, and also 95% of virus-free plants in shoot tip culture. The application of melatonin treatments may provide an alternative means for the eradication of plant viruses and could even be used to produce virus-free plants as an interesting biotechnological approach.
Resistance to
Rice Stripe Virus (RSV) in rice plants treated with melatonin has been studied
[95][142]. The optimum concentration of melatonin and SNP (sodium nitroprusside used as a nitric oxide (NO)-releasing reagent) to reduce disease incidence in the RSV-suscetible
Nipponbare rice cultivar has been screened. The soil of the 14-day-old rice seedling pots was kept as dry as possible, followed by the application of the different treatments, 0.1, 1, 10, and 100 μM of melatonin or 10, 50, 100, 500 and 1000 µM of SNP. Rice seedlings are placed in the dark for 12 h. After that, they were inoculated with RSV for 3 days. The plants were then transferred to the soil in the greenhouse. Thirty plants were used for each treatment. The results showed that both (melatonin and SNP) can reduce disease incidence in a concentration dependent manner, with the largest effect being observed with 10 μM melatonin and 100 μM SNP. Therefore, the application of exogenous melatonin and NO can promote rice resistance to RSV. Additionally, both compounds positively modulated the expression of two genes (OsPR1b and OsWRKY45 involved in the induction of PR genes (pathogenesis related protein)), indicating that melatonin and NO are able to enhance the plant disease-resistance genes in RSV disease. So, Lu et al. (2019) also quantified the endogenous melatonin levels in two rice cultivars,
Nipponbare and
Zhendao-88 (susceptible and resistant cultivars to RSV, respectively) after RSV infection. The data showed that resistance to RSV was improved by increased endogenous melatonin and NO production, and established that melatonin was responsible for rice resistance to RSV infection by inducing NO. The authors conclude that rice resistance to RSV can be improved by increasing melatonin through a NO-dependent pathway. The authors postulate that increased melatonin in the resistant cultivar
Zhendao-88 could lead to more NO, which might lead to more SA, which may be the explanation for the increased resistance of this cultivar to RSV
[95][142].