Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nan Zhou and Version 3 by Conner Chen.
Collagen has been widely applied as a functional biomaterial in regulating tissue regeneration and drug delivery by participating in cell proliferation, differentiation, migration, intercellular signal transmission, tissue formation, and blood coagulation.
- collagen
- pharmacological functions
- biosynthesis
1. Physiological and Therapeutic Effects of Collagens
The main physiological function of collagen is to provide support in connective tissue as a structural protein, especially for collagen I, which has the highest content in the human body. Collagen also has many other biological functions such as binding to integrins and other factors to mediate signal transduction, to connect cells and extracellular matrix biological signals. There is a relationship between a lack of all kinds of collagen and bone disease, skin disease, gastrointestinal disease, vascular disease, kidney disease, neurological diseases, and even cancer, so the potential of collagen in clinical treatment may be still not be fully developed (Figure 12, Table 12).
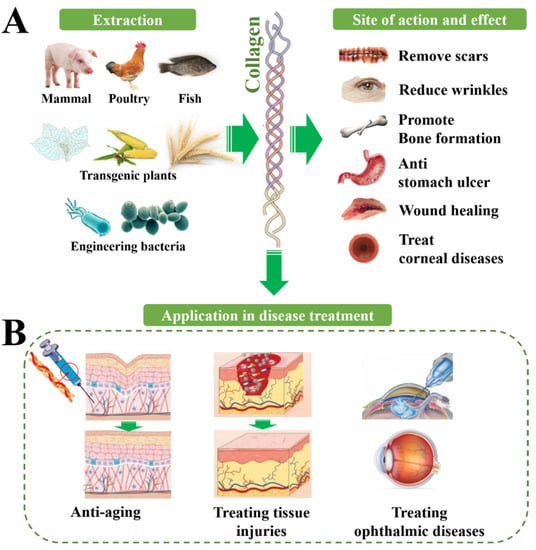
Figure 12. Biological functions of collagen from a variety of sources and their translation into therapeutic application. (A) The therapeutic effect of collagen on different sites, including promoting tissue repair, smoothing wrinkles, promoting bone regeneration, etc. (B) The application of collagen in the treatment of different diseases.
2. Collagen for Treating Skin Injuries
Collagen also plays an important role in regulating the regeneration of tissues, promoting cell adhesion, and cell proliferation. Previous studies show that collagen is closely involved in cell proliferation, differentiation and migration, intercellular signal transmission, tissue formation, blood coagulation, and so on, thus it may participate in different stages of tissue repair. During hemostasis, collagen induces platelet activation and aggregation, thereby depositing fiber clots at the injured site. In the inflammatory stage, the activation of immune cells promotes the secretion of inflammatory cytokines, thus affecting the migration of fibroblasts, epithelial cells, and endothelial cells, and fibroblasts contribute to the deposition of collagen. Collagen degradation releases fragments that promote fibroblast proliferation and growth factor synthesis, leading to angiogenesis and reepithelialization. In the mature remodeling phase, the extracellular matrix is remodeled to restore its tensile strength. So, medical materials based on natural collagen are extensively used as an alternative option to traditional materials, which show non-immunogenicity, low sensitization, and better biocompatibility, and have promising clinical applications [1][13].
Table 12.
Therapeutic application of collagen.
| Diseases | Therapeutic Effects | Refs. | |
|---|---|---|---|
| Treating skin injuries | Wound healing | Wound closure; anti-bacterial activity | [2][3][4][5][6][7][14,15,16,17,18,19] |
| Burn healing | Accelerated healing and skin appendage generation | [8][9][10][11][20,21,22,23] | |
| Chronic wound | Faster wound healing | [12][13][14][24,25,26] | |
| Treating orthopedic diseases | Osteoporosis | Improved bone mineral density; increased bone hydroxyproline content; enhanced alkaline phosphatase level | [15][27] |
| Bone defect healing | New bone tissue forming; guided bone regeneration | [16][[19][2817][18],29,30,31] | |
| Treating ophthalmic diseases | Corneal defects | Filled corneal defects; restored corneal curvature | [17][20][29,32] |
| Keratoconus | Increased corneal rigidity; decreased interfibrillar Bragg spacing | [21][22][23][24][33,34,35,36] | |
| Promoting nerve regeneration | Central nerve injury | Tuning NSCs; improved motor performance; reduced formation of fluid-filled cysts; impeded collapse of musculature and connective tissue | [25][26][27][28][29][30][31][37,38,39,40,41,42,43] |
| Peripheral nerve injury | Well-organized fibers; unimpaired myelin sheath | [32][44] | |
| Anti-aging | Skin anti-aging | Reduced trans-epidermal water loss and skin pore number; increased elasticity; enhanced dermal thickness and acoustic density | [33]45[34][,4635],47[36][,48] |
The wet strength of a collagen sponge allows for suturing of soft tissue and provides a template for the growth of new tissue. Collagen hydrogel was shown to be a potential wet wound dressing that could significantly accelerate the production of new skin appendages [37][49]. Collagen dressings are usually formulated from bovine, avian, or porcine collagen and are easy to apply and remove [9][21]. In addition, collagen dressings can be derived from marine sources. Nile tilapia is one of the most commonly cultured fish in China, and the collagen hydrogel from tilapia skin can be used as a wound dressing for the treatment of deep second-degree burns [38][50]. Oral administration of collagen can also be an effective treatment for wound healing. Studies related to oral jellyfish collagen peptide and salmon skin collagen peptide have shown positive effects on wound healing [8][20].
3. Collagen for Treating Orthopedic Diseases
Collagen also constitutes tendons connecting bone with muscle along with other tissue such as the knee and joints, which makes muscles and bones soft and elastic during exercise. Human bones are mainly made up of calcium and collagen, of which calcium salts account for 2/3 and collagen for 1/3 [37][49]. Osteoporosis is caused by the loss of calcium, and collagen is essential in binding calcium to bone cells and improving osteoporosis; analysis results show that collagen forms a reticular structure in bone, which increases bone stiffness and toughness. Collagen I and collagen II are the main components of hyaline articular cartilage, fibrocartilage, and fibrous tissue [39][40][51,52]. So, collagen II cannot be used only to determine whether the cartilage is hyaline, as it can also be found in fibrocartilage [41][53], and loss of collagen is the cause of heterogeneous genetic disease involving bone fragility deformities, blue sclera, structural shortness, hypodentinogenesis, and hearing loss [42][43][54,55]. In a study, 13-month-old mice were used to evaluate the effects of collagen materials from silver carp skin on osteoporosis, and the results indicated that collagen materials from fish could be applied to alleviate osteoporosis or treat bone loss [15][27]. In application, a thermal-response hydrogel composite composed of triblock PEG-PCL-PEG copolymer, collagen, and nano-hydroxyapatite was developed by ShaoZhi Fu et al. [16][28] and it has shown great potential in bone tissue engineering.
4. Collagen for Treating Ophthalmic Diseases
The corneal stroma is composed mainly of collagen I fibrils organized into ~300 orthogonally arranged lamellas [44][56]. In addition to the stroma, the corneal endothelium consists of a single layer of interconnected hexagonal cells located on Descemet’s membrane. This layer is critical for maintaining relative stroma shedding/dehydration, which is essential for corneal transparency [44][56]. The Descemet’s basement membrane is mainly composed of collagen IV as well as laminin, perlecan (a heparan sulfate proteoglycan), nidogen, and, to a lesser extent, collagen VIII [45][46][57,58]. Collagen is also an important component of the lens, and the lens capsule is structurally similar to Descemet’s membrane in the cornea, as it consists of a network of collagen IV and laminin bound together by nidogen and perlecan [47][59]. The lens capsule serves as a supporting matrix for the epithelial cells of the anterior lens and the fibrocytes of the posterior lens. Since this structure encapsulates all lens cells, it also protects them from infection. In the retina, collagen IV is an abundant component of all basement membranes [48][60], and collagen IV is present in a sandwich form on both sides of Bruch’s membrane, with the middle layer containing elastic fiber transactions that can also be detected in the extracellular matrix surrounding human RPE cells [49][61].
Continuous supplementation of collagen peptide may improve the phenomenon of blurred vision and effectively prevent cataracts, iritis, and other ophthalmic diseases [50][5]. Wollensak et al. [22][34] validated that the crosslinked corneal collagen obtained by riboflavin and UVA irradiation increased the hardness and strength of corneal stroma and prevented the further development of keratoconus. Alternatively, some contact lenses made of collagen have been developed, which can improve the water-holding capacity of the eyes.
5. Collagen for Promoting Nerve Regeneration
Some results of molecular and cellular experiments show that collagen IV in the peripheral extracellular matrix is the key signaling molecule to activate the axon clusters. Collagen VI fulfills a neuroprotective function in the extracellular milieu of the brain that counteracts Aβ-induced neurotoxicity. This function is dependent on the assembly state of collagen VI and may involve interactions with Aβ as well as molecules on the neuronal surface. Collagen VI-related mechanisms may represent novel targets for protective strategies against AD and are supported by NIA and NINDS [48][60]. Collagen IV can interact with the FNIII domain of neural cell adhesion molecule 1 to improve the stability of the latter in the axial membrane and inhibit the lysosomal degradation pathway of neural cell adhesion molecule 1, to promote axonal assembly. The in vivo rat model of sciatic nerve defects showed that increasing the content of collagen IV in the regeneration microenvironment could promote the regeneration of parallel and orderly nerve bundles. At the same time, the projection accuracy and functional recovery of the regenerated nerve were improved [51][62]. In vitro studies by Peiwen Chen et al. [52][63] identified collagen VI as a novel regulator for peripheral nerve regeneration by modulating macrophage function. In practice, a study by Dan Lv et al. [53][64] confirmed that polycaprolactone/collagen VI conduits with sustained release of collagen VI in the local microenvironment may, through triggering macrophage M2 polarization, enhance nerve regeneration. Furthermore, according to Papon Muangsanit et al. [54][65], rat tail collagen I gels were used as a matrix to load human umbilical vein endothelial cells, and the tissue-engineered constructs containing aligned endothelial cells within the collagen matrix could be good candidates to treat peripheral nerve injury.
6. Collagen for Anti-Aging
Collagen in the dermis makes up 70% of the skin composition, it achieves the adhesion of myofibrin in myosin, and can also reach the basal layer of skin through blood circulation to strengthen the skin protection function, elasticity, and firmness; its proper elasticity and hardness facilitate movement of the body [55][66]. Moreover, the basement membrane of the epidermis is tightly combined with the collagen in the dermis, wrapping the sebaceous glands and hair root cells in dermis, thereby propping up the epidermis and dermis and reducing skin wrinkles.
Due to high biocompatibility with the human body, collagen I is the most used collagen in cosmetic production. In recent years, oral collagen supplements have become very popular. According to clinical trials in recent years, oral supplementation of collagen has improved skin hydration, roughness, elasticity, and density, and significantly reduced fragmentation of the collagen network [56][57][67,68]. A study by Chen et al. [58][69] showed that after continuous intake of collagen peptide for 12 weeks, the treatment group had significantly reduced periorbital wrinkles, increased facial skin moisture, and enhanced skin elasticity.
