Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Kyle Kristopher Payne.
The efficacy of current immunotherapies remains limited in many solid epithelial malignancies. Recent investigations into the biology of butyrophilin (BTN) and butyrophilin-like (BTNL) molecules, however, suggest these molecules are potent immunosuppressors of antigen-specific protective T cell activity in tumor beds. BTN and BTNL molecules also associate with each other dynamically on cellular surfaces in specific contexts, which modulates their biology.
- immune oncology
- immunotherapy
- immune suppression
1. Introduction
The dissection of immune checkpoint biology has driven enormous advances in ourthe understanding of how protective T cell activity is regulated in the tumor microenvironment [1]. Immune checkpoint molecules, e.g., PD-1, CTLA-4, B7-H3, B7-H4, drive the paralysis of protective T cells in tumor beds [2,3,4,5,6][2][3][4][5][6]. Consequently, translational research has focused heavily on developing monoclonal antibodies that target these molecules to restore protective T cell activity in cancer [5,6][5][6]. Indeed, several monoclonal antibodies targeting both PD-1 and CTLA-4 have been approved for the treatment of patients with cancer, where they have unquestionably improved the management of several malignancies [1]. However, the efficacy of Pembrolizumab and Ipilimumab in inducing clinically relevant response rates is limited in several solid epithelial malignancies. This includes advanced recurrent ovarian cancers [7] and advanced triple-negative breast cancer, where clinical efficacy is evident only in patients with a PD-L1 combined positive score of 10 or more [8]. Breast and serous ovarian carcinoma are indeed immunogenic and exert clinically relevant antitumor T cell pressure [9,10,11,12][9][10][11][12]. This immunogenicity is likely driven at least in part by genomic instability resulting from defects in homologous recombination repair machinery, which are frequently mutated in breast and ovarian cancers [13,14][13][14]. Despite the immunogenic nature of these malignancies, the current class of immune checkpoint immunotherapeutic approaches has demonstrated limited clinical benefit [7,8][7][8]. The ability of combination CTLA-4/PD-1 checkpoint therapies to restore protective T cell activity appears to be partially dependent on the presence of intraepithelial T cell and myeloid cell niches in ovarian cancer beds [9]. As suggested by others, the development of biomarkers characterizing the tumor-immune phenotype in high-grade serous ovarian cancer may identify patients with a greater likelihood to respond to PD1/PDL1 and CTLA4 blockade [15].
2. Butyrophilins: An Overview
BTN and BTNL molecules are a family of structurally related transmembrane glycoproteins that play important roles in regulating immune responses. In humans, they consist of 13 members; BTN1A1, BTN2A1, BTN2A2, BTN2A3, BTN3A1, BTN3A2, BTN3A3, BTNL2, BTNL3, BTNL8, BTNL9, BTNL10 (a pseudogene), and SKINT-like (SKINTL). The extracellular domains of BTN and BTNL molecules are structurally similar to the B7 family of costimulatory ligands; this includes PD-L1, B7-H3, and B7-H4, further suggesting an immunomodulatory role of these molecules. In limited studies to date, BTN/BTNLs have been shown to be involved in both activating and inhibiting T cell activity under homeostatic conditions as well as during pathological manifestations, such as cancer. Both BTN and BTNL molecules have a broad expression profile, with demonstrated surface expression on various immune cell subsets (Table 1) [18][16], as well as epithelial tissues such as the gut and some cancers; these include breast and ovarian carcinomas [17,19][17][18]. Additionally, polymorphisms of several BTN and BTNL molecules are associated with inflammatory diseases, including myositis and sarcoidosis [20[19][20][21][22],21,22,23], suggesting potential immunoregulatory roles. Indeed, the expression patterns of some BTN and BTNL molecules have been correlated with clinical outcome in patients with cancer [17,24,25][17][23][24]. However, knowledge about the extracellular and intracellular binding partners of many BTN/BTNL molecules and their mechanisms of signaling for any potential immunomodulatory function is still limited. As such, a comprehensive elucidation of the mechanistic activity of BTN/BTNL molecules potentially driving these associations in cancer has the potential to unleash a new class of cancer-directed immunotherapeutics.Table 1. The expression profile of the Butyrophilin family on different immune cell subsets.
| Gene | Predicted Location | The Expression on Immune Cell Subsets |
|---|---|---|
| BTN1A1 | Membrane | Memory B-cell, Naive B-cell, Non-classical monocyte, Eosinophil, MAIT T cells |
| BTN2A1 | Intracellular, membrane | Neutrophil, Basophil, Eosinophil, T-reg, Memory CD8 T-cell, γδ T-cell, Naive CD8 T-cell, NK-cell, MAIT T-cell, Memory CD4 T-cell, Classical monocyte, Plasmacytoid DC, Non-classical monocyte, Naive CD4 T-cell, Memory B-cell, Myeloid DC, Total PBMC, Naive B-cell, Intermediate monocytes |
| BTN2A2 | Intracellular, membrane | Naive B-cell, Memory B-cell, Myeloid DC, Intermediate monocyte, Classical monocyte, Neutrophil, T-reg, Plasmacytoid DC, NK-cell, Total PBMC, Non-classical monocyte, γδ T-cell, MAIT T-cell, Memory CD8 T-cell, Naive CD8 T-cell, Memory CD4 T-cell, Naive CD4 T-cell, Eosinophil, Basophil |
| BTN3A1 | Intracellular, membrane | Basophil, γδ T-cell, Memory CD8 T-cell, NK-cell, T-reg, Naive CD8 T-cell, Eosinophil, Total PBMC, Memory CD4 T-cell, Naive CD4 T-cell, Neutrophil, Memory B-cell, Non-classical monocyte, Intermediate monocyte, Classical monocyte, Naive B-cell, Myeloid DC, Plasmacytoid DC |
| BTN3A2 | Intracellular, membrane | γδ T-cell, Memory CD8 T-cell, T-reg, Naive CD8 T-cell, NK-cell, MAIT T-cell, Naive CD4 T-cell, Memory CD4 T-cell, Total PBMC, Basophil, Memory B-cell, Eosinophil, Naive B-cell, Non-classical monocyte, Neutrophil, Intermediate monocyte, Classical monocyte, Myeloid DC, Plasmacytoid DC |
| BTN3A3 | Intracellular, membrane | Memory CD8 T-cell, γδ T-cell, Basophil, Naive CD8 T-cell, T-reg, NK-cell, MAIT T-cell, Memory CD4 T-cell, Naive CD4 T-cell, Total PBMC, Non-classical monocyte, Intermediate monocyte, Memory B-cell, Classical monocyte, Naive B-cell, Eosinophil, Myeloid DC, Neutrophil, Plasmacytoid DC |
| BTNL2 | Intracellular, membrane | Not detected in Immune cells |
| BTNL3 | Membrane | Neutrophils |
| BTNL8 | Membrane | Neutrophil, Eosinophil |
| BTNL9 | Membrane | Memory B-cell, Naive B-cell, Basophil, Neutrophil, Eosinophil, Classical monocyte |
3. Butyrophilins Regulate Protective Antitumor Immunity
BTN and BTNL molecules have gained the attention of tumor immunologists due to their ability to regulate T cell activity. Butyrophilin-like 2 (BTNL2) has the potential to suppress T cell activation through direct contact with the T cell surface, as chimeric BTNL2-Fc proteins have been demonstrated to impair T cell proliferation and cytokine production [26][25]. Likewise, murine BTNL2 has been demonstrated to function as a potent suppressor of the anti-tumor immune response. The antibody-mediated blockade of BTNL2 attenuated tumor progression in multiple in vivo murine tumor models, resulting in the prolonged survival of tumor-bearing mice [27][26]. Interestingly, studies utilizing murine T cells have shown an additional mechanism of BTNL2-mediated immune suppression where recombinant murine BTNL2 was found to promote T cell-intrinsic Foxp3 expression by eliciting the blockade of the Akt-mediated inactivation of Foxo1, therefore promoting a suppressive, regulatory-like T cell phenotype [28][27]. While the role of BTNL2 in protective immune response is becoming clearer, the functions of other BTNL molecules in anti-tumor immunity require further clarification. For example, BTNL8 is suggested to play an essential role in the priming of naïve T cells, however, it did not enhance the production of memory T cells [39][28]. This suggests BTNL8 may be advantageous in cancer immunotherapy by potentially provoking polyclonal ‘effector-like’ T cell responses, which have been associated with better outcomes for some cancer patients. Moreover, BTNL8 has also recently been reported to drive the activation of subsets of gut-resident γδ T cells through cell surface interactions with BTNL3 [40][29]. While lacking mechanistic investigation, both BTNL3 and BTNL8 recombinant proteins appear to drive the differential regulation of interferon-gamma secretion from CD3+ T cells (e.g., rndsystems.org, accessed 3 May 2023.), suggesting potential contextual implications for their impact on both αβ and γδ T cell functionality. While these molecules are widely expressed in cancer, their impact in this setting remains unknown. Of the butyrophilin-like molecules, BTNL9 remains the most poorly characterized in humans. However, its expression has been positively associated with higher immune infiltration and survival in a cohort of patients with lung adenocarcinoma [38][30] (Table 1), suggesting a possible immune co-stimulatory function. Mechanistically, it was demonstrated that mutant p53 is a significant factor contributing to decreased BTNL9 expression in patients with breast cancer. Intriguingly, in a 2021 study, Mo et al. revealed the impact of BTNL9 expression on the progression of breast cancer through cancer cell-intrinsic regulation of the cell cycle. In tThis study, the elevated expression of BTNL9 blocked breast cancer cells in G2/M via P53/CDC25C and P53/GADD45 pathways [41][31], suggesting that BTNL9 could be a promising biomarker for the early detection of breast cancer, thereby enabling timely and effective treatment. Thus, while BTNL molecules may most logically play a role in immune regulation due to their homology to B7 family members, the potential immune-independent regulation of cancer progression by BTNL molecules may represent an intriguing area of investigation. The activity of BTN molecules also drives immune regulatory activity. BTN2A2 appears to dampen dysregulated T cell activity associated with autoimmunity, as the exogenous administration of BTN2A2-IgG2a Fc proteins ameliorated the pathogenicity of a disease modeling rheumatoid arthritis in mice through the impairment of T cell proliferation and production of Th1/Th17 cytokines [42][32]. Additionally, BTN3A1, while currently appreciated as an activator of Vγ9Vδ2 T cells [40[29][33][34][35],43,44,45], was described over a decade ago as a suppressor of αβ T cells. In 2010, BTN3A1 was found to be widely expressed on immunosuppressive cells found within the ovarian tumor microenvironment and to function as a suppressor of T cell activity by leveraging in vitro studies utilizing K562 cells ‘presenting’ BTN3A1 on their surface [16][36]. Supportive of the immunomodulatory activity of BTN3A1, the concentration of soluble BTN3A1 found in the plasma of metastatic clear cell renal carcinoma patients was found to predict therapeutic responses to the checkpoint immunotherapeutic nivolumab [31][37]. Intriguingly, while the BTN3A family likely has shared functional activity in suppressing αβ T cells due to the nearly identical homology of their extracellular domains, BTN3A3 has also been demonstrated to possess an immunomodulatory function independent of T cells. In fact, xenograft models of breast cancer expressing tumor cell surface BTN3A3 modulated the function of macrophages to promote the acquisition of a cancer stem cell (CSC)-like phenotype [32][38]. Mechanistically, macrophages engaged BTN3A3 through surface expression of the C-type lectin, LSECtin, to promote this phenotype. Either macrophage-specific ablation of LSECtin or silencing of BTN3A3 in breast cancer cells decreased CSC frequency and tumor growth. BTN2A1, furthermore, has been found to engage CD209 (also known as DC-SIGN) on the surface of immature monocytes through high-mannose oligosaccharides [46][39]. More recently, BTN3A1 has been shown to engage CD45 on the surface of αβ T cells through an N-linked glycan-dependent mechanism [17]. Curiously, nearly all BTN and BTNL molecules are predicted to be post-translationally modified with glycosylation motifs [47][40], suggesting these carbohydrate moieties may be important in driving the biology associated with these molecules. Collectively, previous work investigating BTN/BTNL molecules clearly demonstrates their potential to modulate immune cell activity. Clarifying the roles of these molecules in cancer and, subsequently, their potential to function as immunotherapeutic targets may drive the development of more effective cancer immunotherapies, as discussed in the case of BTN3A1 below.4. BTN3A1 Impairs Tumor-Specific αβ T Cells in Cancer
BTN3A1 is most widely appreciated to function as an activator of the Vγ9Vδ2 subset of γδ T cells, in concert with BTN2A1 [40,43,44,45,48][29][33][34][35][41]. Intriguingly, however, it has been reported that BTN3A1 also functions as a regulator of αβ T cells [16,17][17][36]. In a mechanistic interrogation of the impact of BTN3A1 on antitumor immunity, Payne et al. found the dysregulated expression of this molecule in high-grade serous ovarian cancer (HGSOC) and triple-negative breast cancer [17,25][17][24]. While dysregulated expression has been observed on the surface of malignant cells and infiltrating leukocytes—most notably dendritic cells—in both malignancies, the mechanisms driving aberrant overexpression of BTN3A1 in cancer remain unknown [16,17][17][36]. However, this dysregulated expression is likely a result of aberrant NLRC5 activity in these malignancies, as NLRC5 was recently found in the transcription of BTN3A genes through an atypical SXY module within the major histocompatibility complex [49][42]. Importantly, this dysregulated expression of BTN3A1 was found to be inversely correlated with HGSOC patient survival [17]. Critically, in a mechanism which appears to be distinct from its ability to activate Vγ9Vδ2 T cells, recombinant BTN3A1-Fc chimeric proteins were demonstrated to impair the phosphorylation of activating tyrosine residues in signaling molecules proximal to the αβ T cell receptor [17], while K32 cells ectopically expressing BTN3A1 impaired the proliferative potential of purified αβ T cells in vitro [16][36]. By leveraging novel transgenic mice in which BTN3A1 was specifically expressed in dendritic cells, Payne et al. demonstrated an elevated malignant progression which paralleled the decreased infiltration and effector activity of αβ T cells in the setting of ovarian cancer. Targeting BTN3A1 in vivo using CTX-2026, a unique fully human aglycosylated monoclonal antibody delayed the progression of ovarian cancer in both syngenic and xenograft models through the elicitation of more robust αβ T cell responses. Additionally, targeting BTN3A1 with CTX2026 impaired malignant progression more robustly than the PD-1 inhibitor, Nivolumab. Mechanistically, it was found that BTN3A1 engages CD45 of the surface of αβ T cells through an N-linked glycosylation-dependent mechanism independent of BTN2A1; this engagement was found to physically impede the formation of the immune synapse through impairing the segregation of CD45 from the T cell receptor [17,50][17][43] (Figure 1). This restudyearch underscores the importance of BTN3A1 in blunting protective antitumor T cell activity in the setting of cancer, and lays bare the proposition that targeting this immunosuppressive pathway may be more effective in unleashing protective immunity than the blockade of PD-1/PD-L1 in certain malignancies.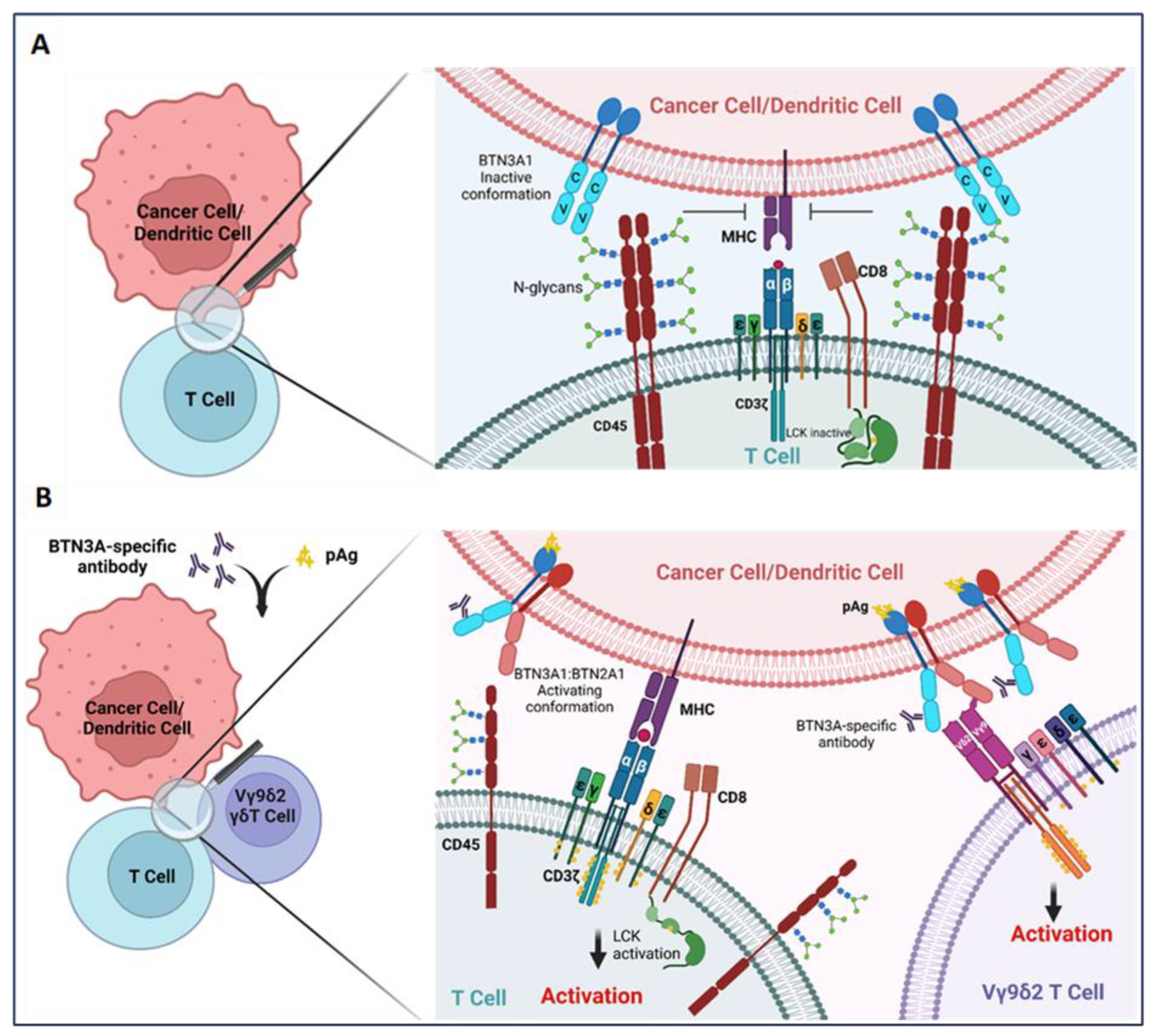
Figure 1. BTN3A1 transformation from an immunosuppressive to an immunostimulatory molecule upon sensing pAgs or anti-BTN3A-specific antibodies. (A) BTN3A1 engagement with N-glycans of CD45 inhibits segregation of N-glycosylated CD45 from the immune synapse on the surface of αβ T cells and prevents the activation of the tumor-reactive T cells; (B) Anti-BTN3A antibodies or pAgs sensed by BTN3A1 drive BTN3A1:BTN2A1 interactions; BTN2A1 binds to the Vγ9 TCR chain and promotes Vγ9Vδ2 subset of γδ T cell activation. Transformation of BTN3A1 from an immunosuppressive molecule (through engagement with N-glycosylated CD45) for tumor antigen-specific T cells to an immunostimulatory molecule also rescues αβ T cell anti-tumor activity.
References
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801.
- Stephen, T.L.; Payne, K.K.; Chaurio, R.A.; Allegrezza, M.J.; Zhu, H.; Perez-Sanz, J.; Perales-Puchalt, A.; Nguyen, J.M.; Vara-Ailor, A.E.; Eruslanov, E.B.; et al. SATB1 Expression Governs Epigenetic Repression of PD-1 in Tumor-Reactive T Cells. Immunity 2017, 46, 51–64.
- Kontos, F.; Michelakos, T.; Kurokawa, T.; Sadagopan, A.; Schwab, J.H.; Ferrone, C.R.; Ferrone, S. B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clin. Cancer Res. 2021, 27, 1227–1235.
- Wang, J.-Y.; Wang, W.-P. B7-H4, a promising target for immunotherapy. Cell. Immunol. 2020, 347, 104008.
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454.
- Weber, J.S.; O’day, S.; Urba, W.; Powderly, J.; Nichol, G.; Yellin, M.; Snively, J.; Hersh, E. Phase I/II Study of Ipilimumab for Patients With Metastatic Melanoma. J. Clin. Oncol. 2008, 26, 5950–5956.
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019, 30, 1080–1087.
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226.
- Duraiswamy, J.; Turrini, R.; Minasyan, A.; Barras, D.; Crespo, I.; Grimm, A.J.; Casado, J.; Genolet, R.; Benedetti, F.; Wicky, A.; et al. Myeloid antigen-presenting cell niches sustain antitumor T cells and license PD-1 blockade via CD28 costimulation. Cancer Cell 2021, 39, 1623–1642.e20.
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213.
- Savas, P.; Virassamy, B.; Ye, C.; Salim, A.; Mintoff, C.P.; Caramia, F.; Salgado, R.; Byrne, D.J.; Teo, Z.L.; Dushyanthen, S.; et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018, 24, 986–993.
- Li, X.; Gruosso, T.; Zuo, D.; Omeroglu, A.; Meterissian, S.; Guiot, M.-C.; Salazar, A.; Park, M.; Levine, H. Infiltration of CD8 + T cells into tumor cell clusters in triple-negative breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3678–3687.
- Min, A.; Kim, K.; Jeong, K.; Choi, S.; Kim, S.; Suh, K.J.; Lee, K.-H.; Kim, S.; Im, S.-A. Homologous repair deficiency score for identifying breast cancers with defective DNA damage response. Sci. Rep. 2020, 10, 12506.
- Mekonnen, N.; Yang, H.; Shin, Y.K. Homologous Recombination Deficiency in Ovarian, Breast, Colorectal, Pancreatic, Non-Small Cell Lung and Prostate Cancers, and the Mechanisms of Resistance to PARP Inhibitors. Front. Oncol. 2022, 12, 880643.
- Kandalaft, L.E.; Laniti, D.D.; Coukos, G. Immunobiology of high-grade serous ovarian cancer: Lessons for clinical translation. Nat. Rev. Cancer 2022, 22, 640–656.
- Uhlen, M.; Karlsson, M.J.; Zhong, W.; Tebani, A.; Pou, C.; Mikes, J.; Lakshmikanth, T.; Forsström, B.; Edfors, F.; Odeberg, J.; et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science 2019, 366, eaax9198.
- Payne, K.K.; Mine, J.A.; Biswas, S.; Chaurio, R.A.; Perales-Puchalt, A.; Anadon, C.M.; Costich, T.L.; Harro, C.M.; Walrath, J.; Ming, Q.; et al. BTN3A1 governs antitumor responses by coordinating αβ and γδ T cells. Science 2020, 369, 942–949.
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102.
- Rhodes, D.A.; Reith, W.; Trowsdale, J. Regulation of Immunity by Butyrophilins. Annu. Rev. Immunol. 2016, 34, 151–172.
- Price, P.; Santoso, L.; Mastaglia, F.; Garlepp, M.; Kok, C.; Allcock, R.; Laing, N. Two major histocompatibility complex haplotypes influence susceptibility to sporadic inclusion body myositis: Critical evaluation of an association with HLA-DR3. Tissue Antigens 2004, 64, 575–580.
- Valentonyte, R.; Hampe, J.; Huse, K.; Rosenstiel, P.; Albrecht, M.; Stenzel, A.; Nagy, M.; Gaede, I.K.; Franke, A.; Häsler, R.; et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat. Genet. 2005, 37, 357–364.
- Rybicki, B.A.; Walewski, J.L.; Maliarik, M.J.; Kian, H.; Iannuzzi, M.C. The BTNL2 Gene and Sarcoidosis Susceptibility in African Americans and Whites. Am. J. Hum. Genet. 2005, 77, 491–499.
- Ren, H.; Li, S.; Liu, X.; Li, W.; Hao, J.; Zhao, N. Multi-omics analysis of the expression and prognostic value of the butyrophilins in breast cancer. J. Leukoc. Biol. 2021, 110, 1181–1195.
- Liang, F.; Zhang, C.; Guo, H.; Gao, S.; Yang, F.; Zhou, G.; Wang, G. Comprehensive analysis of BTN3A1 in cancers: Mining of omics data and validation in patient samples and cellular models. FEBS Open Bio 2021, 11, 2586–2599.
- Arnett, H.A.; Escobar, S.S.; Gonzalez-Suarez, E.; Budelsky, A.L.; Steffen, L.A.; Boiani, N.; Zhang, M.; Siu, G.; Brewer, A.W.; Viney, J.L. BTNL2, a Butyrophilin/B7-Like Molecule, Is a Negative Costimulatory Molecule Modulated in Intestinal Inflammation. J. Immunol. 2007, 178, 1523–1533.
- Du, Y.; Peng, Q.; Du Cheng, D.; Pan, T.; Sun, W.; Wang, H.; Ma, X.; He, R.; Zhang, H.; Cui, Z.; et al. Cancer cell-expressed BTNL2 facilitates tumour immune escape via engagement with IL-17A-producing γδ T cells. Nat. Commun. 2022, 13, 231.
- Swanson, R.M.; Gavin, M.A.; Escobar, S.S.; Rottman, J.B.; Lipsky, B.P.; Dube, S.; Li, L.; Bigler, J.; Wolfson, M.; Arnett, H.A.; et al. Butyrophilin-like 2 Modulates B7 Costimulation To Induce Foxp3 Expression and Regulatory T Cell Development in Mature T Cells. J. Immunol. 2013, 190, 2027–2035.
- Chapoval, A.I.; Smithson, G.; Brunick, L.; Mesri, M.; Boldog, F.L.; Andrew, D.; Khramtsov, N.V.; Feshchenko, E.A.; Starling, G.C.; Mezes, P.S. BTNL8, a butyrophilin-like molecule that costimulates the primary immune response. Mol. Immunol. 2013, 56, 819–828.
- Vantourout, P.; Laing, A.; Woodward, M.J.; Zlatareva, I.; Apolonia, L.; Jones, A.W.; Snijders, A.P.; Malim, M.H.; Hayday, A.C. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology. Proc. Natl. Acad. Sci. USA 2018, 115, 1039–1044.
- Ma, W.; Liang, J.; Mo, J.; Zhang, S.; Hu, N.; Tian, D.; Chen, Z. Butyrophilin-like 9 expression is associated with outcome in lung adenocarcinoma. BMC Cancer 2021, 21, 1096.
- Mo, Q.; Xu, K.; Luo, C.; Zhang, Q.; Wang, L.; Ren, G. BTNL9 is frequently downregulated and inhibits proliferation and metastasis via the P53/CDC25C and P53/GADD45 pathways in breast cancer. Biochem. Biophys. Res. Commun. 2021, 553, 17–24.
- He, X.; Hu, R.; Luo, P.; Gao, J.; Yang, W.; Li, J.; Huang, Y.; Han, F.; Lai, L.; Su, M. BTN2A2 protein negatively regulates T cells to ameliorate collagen-induced arthritis in mice. Sci. Rep. 2021, 11, 19375.
- Vavassori, S.; Kumar, A.; Wan, G.S.; Ramanjaneyulu, G.S.; Cavallari, M.; El Daker, S.; Beddoe, T.; Theodossis, A.; Williams, N.K.; Gostick, E.; et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat. Immunol. 2013, 14, 908–916.
- Sandstrom, A.; Peigné, C.-M.; Léger, A.; Crooks, J.E.; Konczak, F.; Gesnel, M.-C.; Breathnach, R.; Bonneville, M.; Scotet, E.; Adams, E.J. The Intracellular B30.2 Domain of Butyrophilin 3A1 Binds Phosphoantigens to Mediate Activation of Human Vγ9Vδ2 T Cells. Immunity 2014, 40, 490–500.
- Riaño, F.; Karunakaran, M.M.; Starick, L.; Li, J.; Scholz, C.J.; Kunzmann, V.; Olive, D.; Amslinger, S.; Herrmann, T. Vγ9Vδ2 TCR-activation by phosphorylated antigens requires butyrophilin 3 A1 (BTN3A1) and additional genes on human chromosome 6. Eur. J. Immunol. 2014, 44, 2571–2576.
- Cubillos-Ruiz, J.R.; Martinez, D.; Scarlett, U.K.; Rutkowski, M.R.; Nesbeth, Y.C.; Camposeco-Jacobs, A.L.; Conejo-Garcia, J.R. CD277 is a Negative Co-stimulatory Molecule Universally Expressed by Ovarian Cancer Microenvironmental Cells. Oncotarget 2010, 1, 329–338.
- Incorvaia, L.; Fanale, D.; Badalamenti, G.; Porta, C.; Olive, D.; De Luca, I.; Brando, C.; Rizzo, M.; Messina, C.; Rediti, M.; et al. Baseline Plasma Levels of Soluble PD-1, PD-L1, and BTN3A1 Predict Response to Nivolumab Treatment in Patients With Metastatic Renal Cell Carcinoma: A Step Toward a Biomarker for Therapeutic Decisions. OncoImmunology 2020, 9, 1832348.
- Liu, D.; Lu, Q.; Wang, X.; Wang, J.; Lu, N.; Jiang, Z.; Hao, X.; Li, J.; Liu, J.; Cao, P.; et al. LSECtin on tumor-associated macrophages enhances breast cancer stemness via interaction with its receptor BTN3A3. Cell Res. 2019, 29, 365–378.
- Malcherek, G.; Mayr, L.; Roda-Navarro, P.; Rhodes, D.; Miller, N.; Trowsdale, J. The B7 Homolog Butyrophilin BTN2A1 Is a Novel Ligand for DC-SIGN. J. Immunol. 2007, 179, 3804–3811.
- The UniProt Consortium; Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531.
- Rigau, M.; Ostrouska, S.; Fulford, T.S.; Johnson, D.N.; Woods, K.; Ruan, Z.; McWilliam, H.E.; Hudson, C.; Tutuka, C.; Wheatley, A.K.; et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 2021, 367, eeay5516.
- Dang, A.T.; Strietz, J.; Zenobi, A.; Khameneh, H.J.; Brandl, S.M.; Lozza, L.; Conradt, G.; Kaufmann, S.H.; Reith, W.; Kwee, I.; et al. NLRC5 promotes transcription of BTN3A1-3 genes and Vγ9Vδ2 T cell-mediated killing. iScience 2020, 24, 101900.
- Chang, V.T.; Fernandes, R.A.; Ganzinger, A.K.; Lee, S.F.; Siebold, C.; McColl, J.; Jönsson, P.; Palayret, M.; Harlos, K.; Coles, C.H.; et al. Initiation of T cell signaling by CD45 segregation at ‘close contacts’. Nat. Immunol. 2016, 17, 574–582.
More
