Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Marwa Omar.
Neutrophils are the key players in the innate immune system, being weaponized with numerous strategies to eliminate pathogens. The production of extracellular traps is one of the effector mechanisms operated by neutrophils in a process called NETosis. Neutrophil extracellular traps (NETs) are complex webs of extracellular DNA studded with histones and cytoplasmic granular proteins.
- extracellular traps
- protozoa
- innate immunity
- NETosis
- helminths
- neutrophils
- DNA
1. Introduction
Parasitic helminths (metazoa) and protozoa are vastly diverse groups of eukaryotic organisms that cause various diseases in humans and animals. Parasitic infections pose a major public health concern in developing countries, mainly in tropical and subtropical communities [1]. Parasites can cause persistent infection due to their ability to resist immune-mediated expulsion by modulating the host’s immune response [2,3,4][2][3][4]. The intricate interaction between the parasite and the host is critically important when understanding the pathophysiology of the diseases they cause [5].
Innate immunity is an ancient form of the host’s defensive mechanisms against invasive pathogens. This type of immunity has evolved to protect the host against different infectious agents, including bacteria, fungi, viruses, and parasites [6]. The components of the innate machinery include physical and anatomical barriers, along with humoral and cellular factors [7,8][7][8]. Neutrophils, monocytes, and macrophages are among the professional phagocytic cells participating in both phagocytosis and inflammatory processes [9,10][9][10].
The generation of extracellular traps (ETs) has been recognized as a novel mechanism of the innate immune response against infections. These traps are expelled to facilitate the immobilization and killing of invading microorganisms in the extracellular environment [11]. ETs can be generated by several leukocytes, including neutrophils, eosinophils, basophils, and mast cells [12]. In addition, activated T cells, B cells, and monocytes are likely to release mitochondrial DNA (mtDNA), forming extracellular web-like structures. Regardless of the type of cells from which they originate, the traps share a common feature: they consist of nuclear or mtDNA as a backbone, with embedded antimicrobial peptides, histones, and cell-specific proteases [13].
2. Neutrophils and Neutrophil Extracellular Traps (NETs)
2.1. Neutrophils
Neutrophils, or poly-morphonuclear (PMN) leukocytes, are the most abundant cell type circulating in human blood. They are considered the foot soldiers of the immune system and the first comers to the sites of infection or inflammation [14,15][14][15]. Neutrophils play a pivotal role in developing innate and adaptive immune responses [16]. They employ different strategies to combat pathogens, including phagocytosis [17[17][18],18], degranulation [19[19][20],20], and the formation of neutrophil extracellular traps (NETs) [21].
2.2. NETs and NETosis
The original description of NET production was first introduced by Brinkmann et al. (2004) as a new defensive mechanism consisting of the ejection of intracellular material in the form of web-like elements into the surrounding extracellular medium. The authors discovered that when stimulated with phorbol myristate acetate (PMA), lipopolysaccharide (LPS), and interleukin 8 (IL-8), neutrophils release nuclear DNA fibers after exposure to Gram-positive or Gram-negative bacteria [21]. NETs are composed of a backbone of DNA decorated with histones and laced with several cytoplasmic antimicrobial granular proteins [21,22,23][21][22][23]. The granular effector molecules include myeloperoxidase (MPO), neutrophil elastase (NE), lactoferrin, cathepsins, pentraxin, gelatinase, peptidoglycan recognition protein (PRP), calprotectin, bacterial permeability-increasing protein (BPIP), and other leukocyte peptides [24,25,26][24][25][26]. The fibrous DNA strands in the NETs are punctuated with globular protein domains in large aggregates ranging from ~25 nm up to 50 nm in size. The chromatin constitutes the backbone of these structures, as NETs can be degraded by DNases, but not by proteases [27].
The whole process of NET production is called NETosis, a phenomenon first described in the work of Takei et al. (1996) [28], based on the term ‘NET’ [22] and the Greek suffix ‘-osis’ [29]. It is a novel form of cell death that is distinct from autophagy or necrosis, as it is independent of the caspase pathway, and there is no phosphatidylserine exposition on the cell surface [22]. Furthermore, NETosis is not associated with DNA fragmentation, as is the case in apoptosis, but rather with the release of NETs after the disruption of the plasma membrane and nuclear envelope. The sticky DNA-rich networks not only entrap invading pathogens, but eventually kill them [30].
Various reports have defined NETosis as an NADPH oxidase (NOX)-dependent mechanism [21,31][21][31]. Under stimulation, neutrophils generate reactive oxygen species (ROS) by NADPH oxidase-2 (NOX-2) [32]. ROS modulate the granular enzyme MPO, and both are required for the release of NE from granules and its translocation to the nucleus [33,34][33][34]. In the nucleus, NE contributes to chromatin decondensation by the proteolysis of histones. Such events result in the nuclear extrusion of DNA and NET production [22,32][22][32]. Yet, other studies have described a (NOX)-independent NET formation process, which is facilitated through calcium influx and mitochondrial ROS production [35,36][35][36]. Hence, this type of NET expulsion can be called “mitochondria-dependent NETosis” [37].
2.3. Mechanisms of NET Formation
There are at least three different mechanisms by which NETs are formed: classical or suicidal NETosis, the noncanonical pathway, and vital NETosis. The lytic or suicidal type usually occurs slowly (2−4 h) and involves the rupture of the neutrophil plasma membrane. On the other hand, nonlytic or vital NETosis occurs rapidly (within minutes) and does not involve disruption to the plasma membrane or cell lysis [38]. During vital NETosis, neutrophils are still capable of functions such as migration, degranulation, and phagocytosis while casting their sticky filaments [39]. The noncanonical pathway is a new pathway of lethal NETosis that has recently been described. This sort suppresses bacterial residence in the neutrophil cytosol and prevents in vivo microbial dissemination [40].
2.4. Microbial Triggers of NETosis
Multiple stimuli promote the release of NETs. These include the interaction of neutrophils with other immune cells (platelets) after activation with cytokines (IL-8) that help to entrap and eliminate pathogens [41]. Additionally, neutrophils induce vital NETosis when exposed either in vitro or in vivo to whole microorganisms or their proteins [39]. The microbicidal effects of NETs have been confirmed in several human and animal models with bacterial [21], viral [42], and fungal infections [43].
NETs entrap many types of bacterial pathogens to prevent their spread. A variety of Gram-positive and Gram-negative bacteria have been shown to induce NET expulsion. Infection with the swine pathogen Streptococcus suis leads to the signaling of NET formation pathways in a NOX-dependent approach [44]. Additionally, the Gram-negative bacterium Klebsiella pneumoniae proved to be a good inducer of NETosis in a mouse lung infection model [45]. Yet, some bacteria have developed strategies to resist their capture or elimination by the NET structures. Staphylococcus aureus produces different enzymes to interfere with the antimicrobial properties of NETs, such as a nuclease (Nuc), a DNA binding protein, and the extracellular adherence protein (Eap) [46]. These enzymes facilitate the escape from the NET filaments, delay bacteria clearance, and increase the mortality caused by the infection [47]. Besides the reported role of bacteria in triggering NETosis, increasing evidence indicates that viruses can also promote NET formation, which may either promote or prevent viral-induced pathology [48]. In the case of Human Immunodeficiency Virus-1 (HIV-1), NETs promote pathogen clearance through the actions of histones and MPO [49]. Recently, Veras et al. [50] demonstrated an increase in the quantity of the NET components (DNA-MPO complex) in the plasma, tracheal aspirate, and autopsy lung tissues of COVID-19 patients. The study highlighted a possible detrimental role of NETs in the pathophysiology of COVID-19.
Neutrophils and lymphocytes are closely related to the pathophysiology of several inflammatory disorders. The neutrophil-to-lymphocyte ratio (NLR) is a biomarker that conjugates two arms of the immune system: the innate immune response, mainly due to neutrophils, and adaptive immunity, promoted by lymphocytes. This ratio is characterized by an increase in neutrophils and a decline in lymphocytes [51]. In their research, Regolo et al. (2022) tried to assess the prognostic value of (NLR) as a predictor of the outcome of COVID-19 patients. According to them, patients with higher NLR showed a higher risk of intra-hospital mortality and disease progression [52].
The filamentous fungus Candida albicans (C. albicans) is the most widely discussed fungal pathogen in the field of NET release. Following the interaction of neutrophils with C.albicans, the granular protein (NE) is released into the cytoplasm to initiate the production of NETs, which have been shown to kill both yeast and hyphal forms of the fungus [43]. Yet, C. albicans has its unique way of resisting NETs. It potentially modulates the formation of NETs by arresting their proteinaceous components, including elastase, MPO, and histones. Additionally, adhesins on the fungal surface can adsorb the NETs proteins and increase the pathogen’s potency in host tissue destruction [53].
3. NETosis in Parasitic Infections
The functional responses of neutrophils to parasitic infections continue to be uncovered. It has been suggested that the size of the stimulating particle is one of the factors driving the decision of neutrophils to generate NETs instead of phagocytosis. While large particles, such as parasites, can induce the formation of NETs, small particles, such as bacteria, yeast, or viruses, can be eliminated by phagocytosis [54,55][54][55]. Knowledge is not yet abundant about the involvement of NETs in the host’s innate response against metazoan and protozoan parasites [5] (Figure 1).
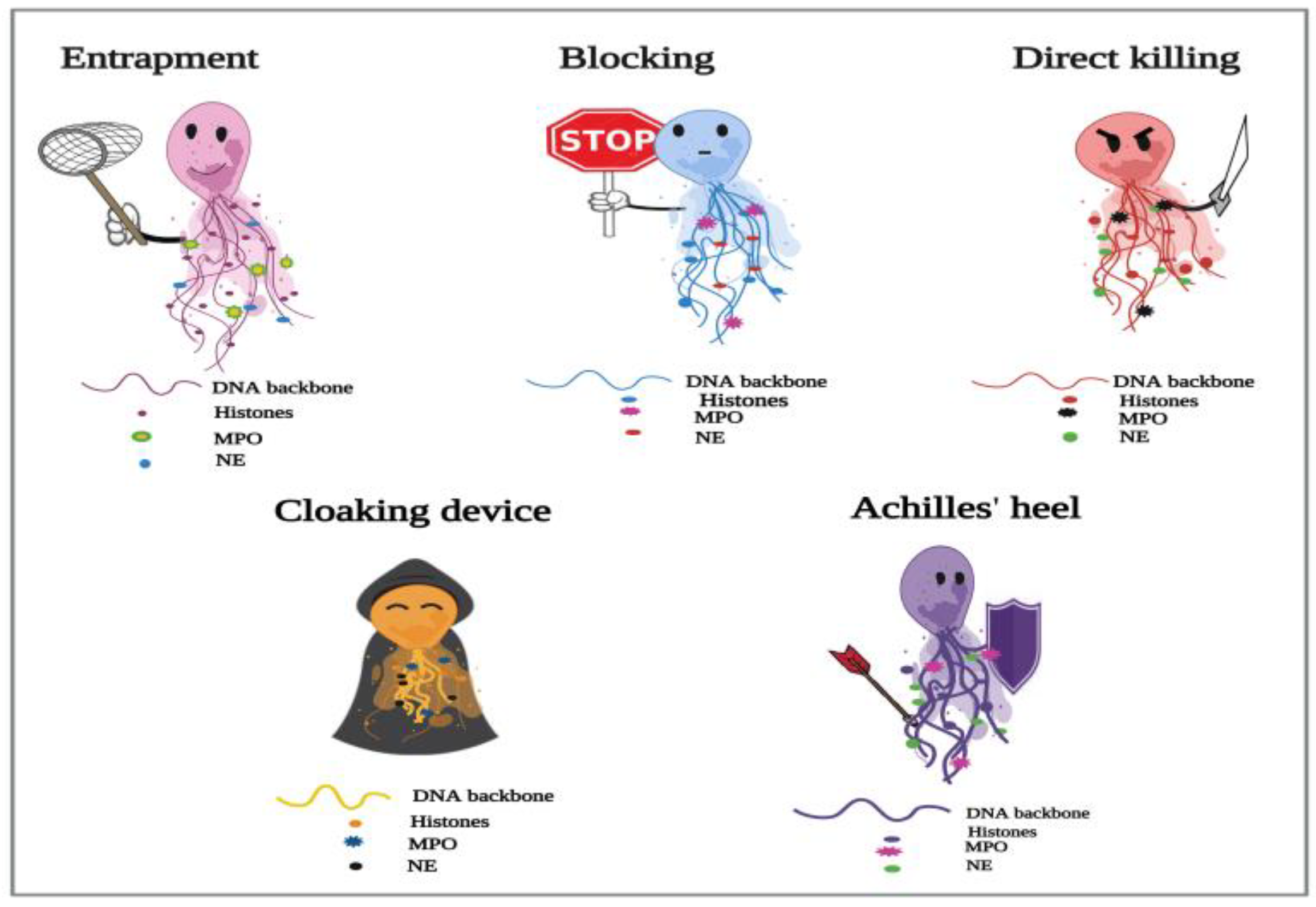
Figure 1. Proposed depiction of the functional responses of NETs to parasitic infections. In the process of NETosis, neutrophils release complex DNA webs, in which actions extend from parasitic entrapment to blocking the worm development and effector killing. Further, NETs could serve as a “cloaking device” to control the spread of infection. WThe aresearchers also have created the “Achilles’ heel of NETs” to frame the several reported limitations in their actions in answer to different parasites.
3.1. NETosis and Metazoan Infections
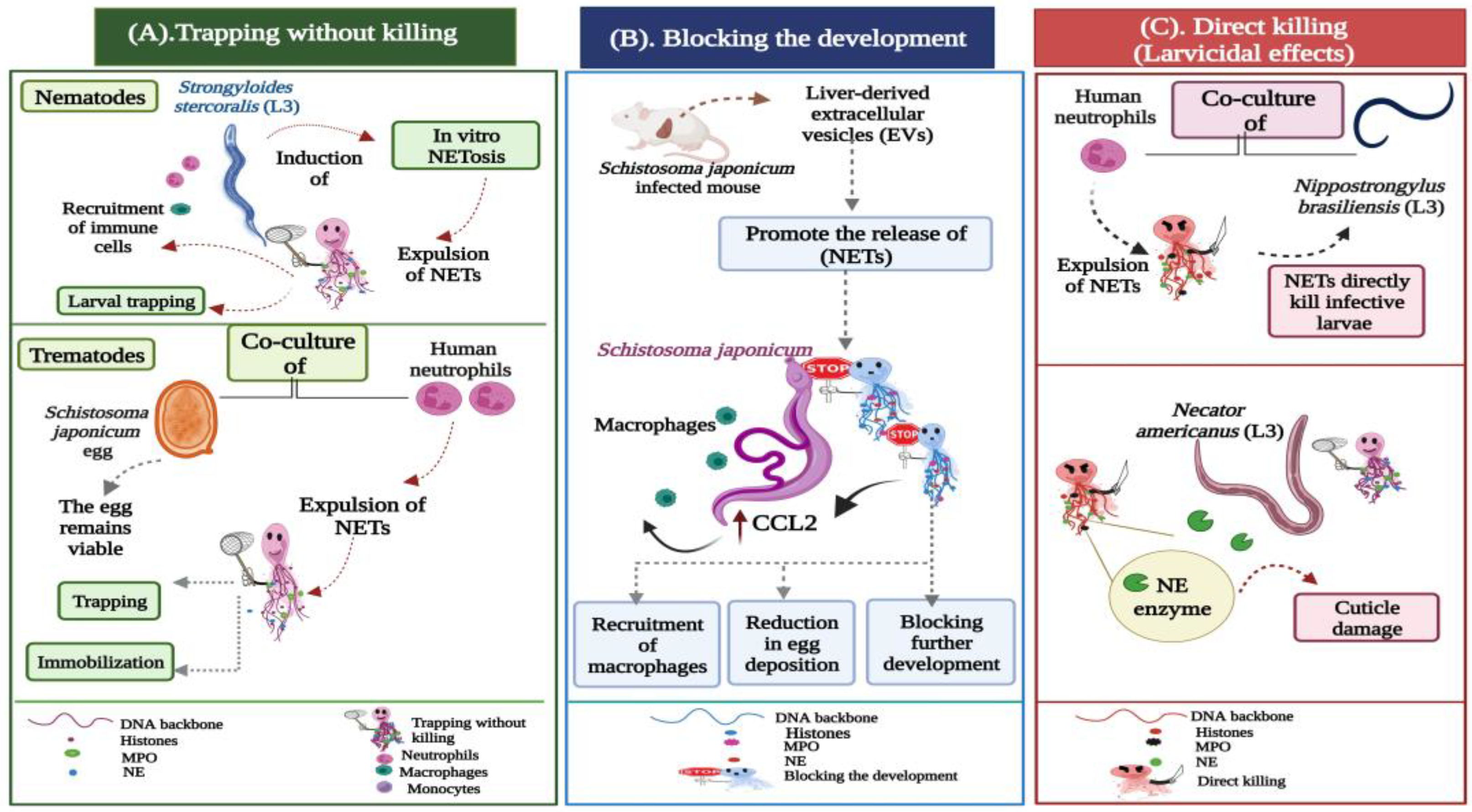
Figure 2. Potential actions operated by NETs during metazoan infections. (A) Trapping without killing: Strongyloides stercoralis large and motile larvae are potent inducers of in vitro NETosis. During the infection, the DNA threads ensnare larvae to enhance their killing by the recruited leukocytes, neutrophils, and macrophages. In trematode-triggered NETosis, the NET filaments capture the egg stages of Schistosoma japonicum. NETs hinder the mobility of the eggs, which still remain viable and intact within the fibrous traps. (B) Blocking the development: Extracellular vesicles (EVs) isolated from the liver of Schistosoma japonicum-infected mice initiate the expulsion of NETs, which block worm development, resulting in a significant reduction in egg deposition and associated fibrosis. The extruded NETs also promote chemokine ligand 2 (CCL2) expression in macrophages, which adhere to the surface of Schistosoma japonicum worms, inhibiting their further development. (C) Direct larvicidal effects: The direct toxicity of neutrophils is distinctly exhibited against the infective stages of hookworms. The skin-penetrating third-stage larvae (L3) of Nippostrongylus brasiliensis and Necator americanus become mechanically trapped by NETs, which directly participate in the larval killing using the neutrophil elastase (NE) enzyme, which induces cuticle damage to the hookworm larvae.
-
Trapping without killing;
-
Blocking the development;
-
Direct killing (larvicidal) effects.
3.2. NETosis and Protozoan Infections
When compared to the studies conducted on other microbial pathogens, the role of NET production in answer to protozoans still seems to be underrepresented. The process of NETosis has been identified during infections with Eimeria, Toxoplasma, Trypanosoma, Plasmodium, and Leishmania parasites [77,78,79,80,81,82][57][58][59][60][61][62]. Yet, until now, less has been known about the multitude of functions that the DNA networks might play during the invasion of host tissues by different protozoa. The positive roles of NETs against protozoa are balanced by their negative impacts on the health of the infected hosts [83][63]. The associations of NETs with inflammatory reactions observed in established protozoan infections raise the possibility of their participation in pathology instead of protection [84][64].References
- Harper, S.L.; Edge, V.L.; Schuster-Wallace, C.J.; Berke, O.; McEwen, S.A. Weather, water quality and infectious gastrointestinal illness in two inuit communities in Nunatsiavut, Canada: Potential implications for climate change. Eco Health 2011, 8, 93–108.
- Chabé, M.; Lokmer, A.; Ségurel, L. Gut protozoa: Friends or foes of the human gut microbiota? Trends Parasitol. 2017, 33, 925–934.
- Burrows, K.; Ngai, L.; Wong, F.; Won, D.; Mortha, A. ILC2 activation by protozoan commensal microbes. Int. J. Mol. Sci. 2019, 20, 4865.
- Ryan, S.M.; Eichenberger, R.M.; Ruscher, R.; Giacomin, P.R.; Loukas, A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog. 2020, 16, e1008508.
- Díaz-Godínez, C.; Carrero, J.C. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. Biosci. Rep. 2019, 39, BSR20180916.
- Medzhitov, R.; Janeway, C., Jr. Innate Immunity. N. Engl. J. Med. 2000, 343, 338–344.
- De Veer, M.J.; Kemp, J.M.; Meeusen, E.N. The innate host defense against nematode parasites. Parasite Immunol. 2007, 29, 1–9.
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503.
- Rossi, M.; Young, J.W. Human dendritic cells: Potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 2005, 175, 1373–1381.
- Liu, K.; Nussenzweig, M.C. Origin and development of dendritic cells. Immunol. Rev. 2010, 234, 45–54.
- Yousefi, S.; Simon, D.; Stojkov, D.; Karsonova, A.; Karaulov, A.; Simon, H.U. In vivo evidence for extracellular DNA trap formation. Cell Death Dis. 2020, 11, 300.
- Simon, D.; Simon, H.U.; Yousefi, S. Extracellular DNA traps in allergic, infectious, and autoimmune diseases. Allergy 2013, 68, 409–416.
- Von Köckritz-Blickwede, M.; Nizet, V. Innate immunity turned inside-out: Antimicrobial defense by phagocyte extracellular traps. J. Mol. Med. 2009, 87, 775–783.
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670.
- Silva, L.M.; Muñoz-Caro, T.; Burgos, R.A.; Hidalgo, M.A.; Taubert, A.; Hermosilla, C. Far beyond phagocytosis: Phagocyte-derived extracellular traps act efficiently against protozoan parasites in vitro and in vivo. Mediat. Inflamm. 2016, 10, 5898074.
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353.
- Hartmann, P.; Becker, R.; Franzen, C.; Schell-Frederick, E.; Romer, J.; Jacobs, M.; Fätkenheuer, G.; Plum, G. Phagocytosis and killing of Mycobacterium avium complex by human neutrophils. J. Leukoc. Biol. 2001, 69, 397–404.
- Lee, W.L.; Harrison, R.E.; Grinstein, S. Phagocytosis by neutrophils. Microbes Infect. 2003, 5, 1299–1306.
- Wazny, T.K.; Mummaw, N.; Styrt, B. Degranulation of human neutrophils after exposure to bacterial phospholipase C. Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 830–832.
- Lacy, P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98–108.
- Brinkmann, V.; Reichard, U.; Goos-mann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535.
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241.
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 2012, 198, 773–783.
- Khan, M.A.; Palaniyar, N. Transcriptional firing helps to drive NETosis. Sci. Rep. 2017, 7, 41749.
- Chapman, E.A.; Lyon, M.; Simpson, D.; Mason, D.; Beynon, R.J.; Moots, R.J.; Wright, H.L. Caught in a trap? Proteomic analysis of neutrophil extracellular traps in rheumatoid arthritis and systemic lupus erythematosus. Front. Immunol. 2019, 10, 423.
- Petretto, A.; Bruschi, M.; Pratesi, F.; Croia, C.; Candiano, G.; Ghiggeri, G.; Migliorini, P. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS ONE 2019, 14, e0218946.
- Brinkmann, V.; Zychlinsky, A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007, 8, 577–582.
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996, 59, 229–240.
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death. Cell Death Differ. 2018, 25, 486–541.
- Guimarães-Costa, A.B.; Nascimento, M.T.; Wardini, A.B.; Pinto-da-Silva, L.H.; Saraiva, E.M. ETosis: A microbicidal mechanism beyond cell death. J. Parasitol. Res. 2012, 2012, 929743.
- Wartha, F.; Henriques-Normark, B. ETosis: A novel cell death pathway. Sci. Signal. 2008, 1, pe25.
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147.
- Bjornsdottir, H.; Welin, A.; Michaelsson, E.; Osla, V.; Berg, S.; Christenson, K.; Sundqvist, M.; Dahlgren, C.; Karlsson, A.; Bylund, J. Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Rad. Biol. Med. 2015, 89, 1024–1035.
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular mechanisms of NETosis. Ann. Rev. Cell Dev. Biol. 2020, 36, 191–218.
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822.
- Villagra-Blanco, R.; Silva, L.M.R.; Gärtner, U.; Wagner, H.; Failing, K.; Wehrend, A.; Taubert, A.; Hermosilla, C. Molecular analyses on Neospora caninum-triggered NETosis in the caprine system. Dev. Comp. Immunol. 2017, 72, 119–127.
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular mechanisms, role in physiology and pathology. Biochemistry 2020, 85, 1178–1190.
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794.
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.V.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393.
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671.
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469.
- Wardini, A.B.; Guimaraes-Costa, A.B.; Nascimento, M.T.; Nadaes, N.R.; Danelli, M.G.; Mazur, C.; Benjamim, C.F.; Saraiva, E.M.; Pinto-da-Silva, L.H. Characterization of neutrophil extracellular traps in cats naturally infected with feline leukemia virus. J. Gen. Virol. 2010, 91, 259–264.
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006, 8, 668–676.
- Ma, F.; Chang, X.; Wang, G.; Zhou, H.; Ma, Z.; Lin, H.; Fan, H. Streptococcus suis serotype 2 stimulates neutrophil extracellular traps formation via activation of p38 MAPK and ERK1/2. Front. Immunol. 2018, 9, 2854.
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell. Biol. 2010, 3, 677–691.
- Chavakis, T.; Wiechmann, K.; Preissner, K.T.; Herrmann, M. Staphylococcus aureus interactions with the endothelium: The role of bacterial “secretable expanded repertoire adhesive molecules” (SERAM) in disturbing host defense systems. Thromb. Haemost. 2005, 94, 278–285.
- Schultz, B.M.; Acevedo, O.A.; Kalergis, A.M.; Bueno, S.M. Role of extracellular trap release during bacterial and viral infection. Front. Microbiol. 2022, 13, 798853.
- Jenne, C.N.; Wong, C.H.; Zemp, F.J.; McDonald, B.; Rahman, M.M.; Forsyth, P.A.; McFadden, G.; Kubes, P. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe 2013, 13, 169–180.
- Saitoh, T.; Komano, J.; Saitoh, Y.; Misawa, T.; Takahama, M.; Kozaki, T.; Uehata, T.; Iwasaki, H.; Omori, H.; Yamaoka, S.; et al. Neutrophil extracellular traps mediate a host defense response to Human Immunodeficiency Virus-1. Cell Host Microbe 2012, 12, 109–116.
- Veras, F.P.; Pontelli, M.C.; Silva, C.M.; Toller-Kawahisa, J.E.; de Lima, M.; Nascimento, D.C.; Schneider, A.H.; Caetité, D.; Tavares, L.A.; Paiva, I.M.; et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020, 217, e20201129.
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636.
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022, 11, 2235.
- Karkowska-Kuleta, J.; Smolarz, M.; Seweryn-Ozog, K.; Satala, D.; Zawrotniak, M.; Wronowska, E.; Bochenska, O.; Kozik, A.; Nobbs, A.H.; Gogol, M.; et al. Proteinous components of neutrophil extracellular traps are arrested by the cell wall proteins of Candida albicans during fungal infection, and can be used in the host invasion. Cells 2021, 10, 2736.
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025.
- Warnatsch, A.; Tsourouktsoglou, T.D.; Branzk, N.; Wang, Q.; Reincke, S.; Herbst, S.; Gutierrez, M.; Papayannopoulos, V. Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity 2017, 46, 421–432.
- Guo, A.J.; Wang, L.; Meng, X.L.; Zhang, S.H.; Sheng, Z.A.; Wei, Z.K.; Luo, X.N.; Huang, W.Y.; Zhu, X.Q.; Zhang, X.C.; et al. Newly excysted juveniles of Fasciola gigantica trigger the release of water buffalo neutrophil extracellular traps in vitro. Exp. Parasitol. 2020, 211, 107828.
- Behrendt, J.H.; Ruiz, A.; Zahner, H.; Taubert, A.; Hermosilla, C. Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria Bovis. Vet. Immunol. Immunopathol. 2010, 133, 1–8.
- Abi Abdallah, D.S.; Lin, C.; Ball, C.J.; King, M.R.; Duhamel, G.E.; Denkers, E.Y. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 2012, 80, 768–777.
- Ponte-Sucre, A. An overview of Trypanosoma brucei infections: An intense host-parasite interaction. Front. Microbiol. 2016, 7, 2126.
- De Buhr, N.; Bonilla, M.C.; Jimenez-Soto, M.; Von Köckritz-Blickwede, M.; Dolz, G. Extracellular trap formation in response to Trypanosoma cruzi infection in granulocytes isolated from dogs and common opossums, natural reservoir hosts. Front. Microbiol. 2018, 9, 966.
- Kho, S.; Minigo, G.; Andries, B.; Leonardo, L.; Prayoga, P.; Poespoprodjo, J.R.; Kenangalem, E.; Price, R.N.; Woodberry, T.; Anstey, N.M. Circulating neutrophil extracellular traps and neutrophil activation are increased in proportion to disease severity in human malaria. J. Infect. Dis. 2019, 219, 1994–2004.
- Pereira, M.A.; Alexandre-Pires, G.; Camara, M.; Santos, M.; Martins, C.; Rodrigues, A.; Adriana, J.; Passero, L.F.D.; Pereira da Fonseca, I.; Santos-Gomes, G. Canine neutrophils cooperate with macrophages in the early stages of Leishmania infantum in vitro infection. Parasite Immunol. 2019, 41, e12617.
- Niedźwiedzka-Rystwej, P.; Repka, W.; Tokarz-Deptuła, B.; Deptuła, W. “In sickness and in health” how neutrophil extracellular trap (NET) works in infections, selected diseases and pregnancy. J. Inflamm. 2019, 16, 15.
- Tabrizi, Z.A.; Khosrojerdi, A.; Aslani, S.; Hemmatzadeh, M.; Babaie, F.; Bairami, A.; Shomali, N.; Hosseinzadeh, R.; Safari, R.; Mohammadi, H. Multi-facets of neutrophil extracellular trap in infectious diseases: Moving beyond immunity. Microb. Pathog. 2021, 158, 105066.
More
