Anaerobic digestion (AD) process is usually limited by factors such as process inefficiencies from substrate-induced instability, poor quality digestate, and poor management of effluent and emissions. Biochar, which is a highly porous carbonaceous material produced from the pyrolysis of biomass is an affordable accelerant that can be used to improve the efficiency of the AD process. Biochar has large specific surface area (SSA), high porosity, and abundant surface functional groups which allows for adsorption and ion exchange thereby removes free ammonia and ions and curbs their deleterious effect on the AD process. The performance of biochar in the AD process usually depends on its properties which are a function of pyrolysis conditions and the type of feedstock used in its production. However, these conditions/properties are not well understood. Additionally, the various mechanisms through which biochar enhances the performance of anaerobic digestion process. Accordingly, this research provides valuable insights into the utilization of biochar as an accelerator to enhance the AD process, addressing crucial factors such as optimal pyrolysis conditions, biochar dosage, and feedstock selection. Additionally, it delves into the mechanisms through which biochar positively influences the AD process, particularly by influencing microbial communities, reducing VFA levels, and mitigating the negative effects of ammonia. The research also highlights areas for further research in applying biochar for anaerobic digestion.
- biochar
- biomass
- greywater
- contaminant removal
- adsorption
- pyrolysis
1. Introduction
Climate change, the energy crisis, resource scarcity, and environmental pollution are critical challenges that will confront humanity in the next decade [1][2]. Two significant contributors to these issues are inadequate solid waste management and excessive reliance on fossil fuels. Various waste-to-energy techniques such as anaerobic digestion (AD), pyrolysis, gasification, and incineration offer double-barreled solution to these challenges through effective management of solid waste and reduction in fossil fuel dependence [3]. Among the abovementioned technologies, the bio-based process of anaerobic digestion (AD) stands out as having the least negative environmental impact [4]. AD involves the decomposition of organic materials by a consortium of bacteria and archaea to produce biogas, which can be further refined into biomethane, and nutrient-rich digestate [5]. However, practical implementation of AD often faces challenges due to the accumulation of volatile fatty acids (VFA), ammonia, and heavy metals, leading to process instability and low biogas production rates, often influenced by the nature of the substrate [6]. While techniques such as the addition of bases to mitigate acidification, two-phase AD, and co-digestion have been employed to address inhibition and enhance the AD process, they do not comprehensively overcome all the challenges [7]. Therefore, alternative solutions are required to improve the applicability of AD in waste management.
Biochar, a solid byproduct obtained from the thermochemical conversion of biomass in the absence of oxygen, serves as a versatile catalyst that can enhance the efficiency of the AD process. Biochar accelerates the methanogenesis stage, safeguards microorganisms against toxic shocks, and reduces the inhibition caused by ammonia and volatile fatty acids, thereby improving methane production. Additionally, biochar can be utilized for in situ purification of biogas to remove carbon dioxide. The effectiveness of biochar in the AD process is influenced by its properties, which depend on the pyrolysis conditions and the type of feedstock used during production. These properties also determine the mechanisms through which biochar enhances the AD process.
2. Factors which influence the efficiency of biochar in the AD process
- Pyrolysis temperature
Pyrolysis temperature is the most relevant factor that influences biochar’s properties as it affects the yield, specific surface area (SSA), pH, type, and amount of surface functional groups in biochar[8]. Various experiments have been carried out to investigate the impact of temperature on the composition of biochar, and it has been noted that high temperature brings about high SSA, low cation exchange capacity (CEC), high pH, reduced yield, and high carbon fractions[9]. In addition, the temperature at which pyrolysis is conducted has an influence on the elemental composition of biochar. Higher temperatures increase the carbon percentage[10]. The substantial differences in hydrogen (H) and oxygen (O) contents after pyrolysis have been ascribed to the cleaving of heterocyclic compounds and nitrile groups at high temperatures[11]. From the aforementioned, it can be seen that conducting pyrolysis at high temperatures can either be beneficial or detrimental to biochar yield. Thus, in pyrolyzing biomass for the AD process, the choice of temperature should be based on the intended purpose of biochar in anaerobic digestion[12]. However, Tripathi et al. [13] stated that to make the process economical, temperatures between 450 and 600 °C are the most suitable based on the type of substrate that suits the purpose of biochar production
- Biochar dosage
Several authors have noted that increasing the quantity of biochar used in anaerobic digestion increases the efficiency of the process, but extremely high doses have detrimental effects on the efficiency of the process[14][15]. The decline in digestion performance at high dosage levels can be attributed to inhibition caused by elevated concentrations of alkali-based metals exceeding acceptable limits and the disruption of microbial networks' diversity[16]. For instance, Paritosh et al.[17] [76] conducted an experiment using hardwood biochar (HBC) at varying doses (5, 10, 15, 20, 25, and 30 g/L) in the digestion of wheat straw. They observed that compared to the control group without HBC (110 L/kg VS), the optimal biochar dosage of 10 g/L doubled the methane yield (223 L/kg VS). However, dosing the digester with biochar beyond 10 g/L resulted in a decrease in methane production. With 15, 20, 25, and 30 g/L HBC, the methane yields were 179.6, 160.0, 148.8, and 149.8 L/kg VS, respectively. In another study by Linville et al.[18], walnut shell biochar was added to a digester during the anaerobic digestion of food waste, with different concentrations (0.96, 1.91, and 3.83 g biochar/g VS added). The addition of biochar increased the methane yield (ranging from 77.5% to 98.1% CH4) compared to the control digester. However, very high dosages (3.83 g biochar/g VS added) resulted in cation toxicity in the digester. These findings highlight the importance of optimizing the dosage of biochar in anaerobic digestion. While increasing the biochar quantity can enhance the process efficiency, exceeding certain threshold levels can have negative effects on methane production due to alkali metal concentrations and cation toxicity. Therefore, careful consideration of the appropriate dosage is necessary to achieve the desired improvements in AD performance while avoiding potential drawbacks.
- Feedstock type
Biochar can be produced from various biomass sources, including plant materials, manure, sludge, and agricultural/food processing residues[19]. The choice of feedstock for biochar production directly influences its yield, chemical composition, and physical properties, which, in turn, impact its effectiveness in the anaerobic digestion (AD) process. Generally, plant and wood-based feedstocks that are rich in lignin and fixed carbon content yield biochar with high specific surface area (SSA), a fine aromatic structure, and low ash content[20]. On the other hand, animal manure and sludge, which have high mineral content, result in biochar with elevated ash content and reduced surface structure and functional groups[21]. The higher SSA of wood-based biochar promotes interspecies electron transfer and accelerates the AD process, making it more efficient in direct interspecies electron transfer (DIET) compared to biochar derived from animal manure or sewage. Moreover, plant-based biochar maintains its cell structure and contains interconnected pores, ranging from 5 to 10 µm in diameter. These abundant pores provide a favorable environment for the entrapment and immobilization of microorganisms, thereby enhancing digestion [22]. All these properties give plant/wood-based biochar an edge over manure or sludge biochar. Though plant/wood-based biochar has a large SSA and high porosity, its ash content is low, and the concentration of alkaline metals—calcium (Ca), magnesium (Mg), sodium (Na), and potassium (K)—in it is low, leading to less alkalinity and a lower ability to prevent acidification in the AD process (from the buildup of volatile fatty acids)[23]. Thus, compared to plant/wood-based biochar, the use of manure or sewage biochar will make the biochar more basic, thereby mitigating ammonia inhibition and increasing the tolerance of the microbial community to acidity. Another important factor to take into consideration with regards to the composition of the parent material is the presence of surface functional groups, thereby enabling direct interspecies electron transfer. Biochar’s EC and abundant surface functional groups increase the methane production rate through direct or indirect electron transfer mechanisms by anaerobes [83,84]. In general, livestock manure and sewage sludge biochar have higher nitrogen (N) and sulfur (S) contents than biochar derived from plants, which are richer in carbon. From the aforementioned, it can be inferred that in choosing feedstock for the production of biochar (that will be used in the anaerobic digester), the availability of the feedstock as well as the aim of the digestion process should be given due consideration.
3. Mechanisms through which biochar improves the performance of the AD process
- Mitigation of ammonia inhibition
The production of ammonia nitrogen during anaerobic digestion (AD) can provide nutrients and partial alkalinity, but excessive levels of ammonia inhibit the AD process[24]. High concentrations of ammonia can penetrate bacterial cell membranes, disrupt proton balances and intracellular pH, and hinder enzymatic activities, thereby impairing the effectiveness of anaerobic digestion. Introducing biochar into the AD process can enhance its tolerance to elevated ammonia and nitrogen levels[25]. Biochar not only acts as a catalyst to accelerate anaerobic digestion but also provides a surface for the growth of microorganisms and the formation of biofilms, which effectively mitigate ammonia inhibition compared to suspended microorganisms[26] (see Figure 1). Furthermore, the surface functional groups of biochar interact with ammonia, rendering it unreactive[27]. Research has demonstrated that black carbon, which includes biochar, can chemically react with nitrogen compounds and convert them[28]. The oxygen groups present on the biochar surface can react with adsorbed ammonia, converting it into amines and amides even under ambient conditions[29]. These amines and amides are typically resistant to degradation, resulting in their stability.
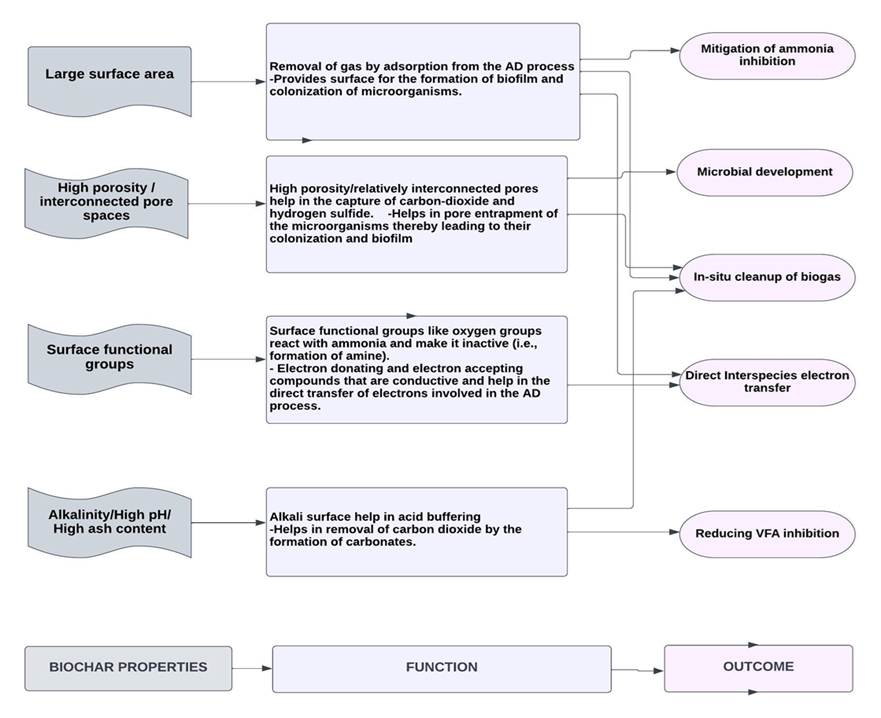
Figure 1: Roles of biochar in the AD process
- Volatile fatty acid reduction
The hydrolysis stage of AD leads to the formation of numerous organic acids, the accumulation of these acids (especially VFAs) lowers the pH of the digestion process and inhibits methanogens, especially at high organic loadings[30]. In addition, the high concentration of the acids can cause their penetration into the cell membrane and subsequent damage to macromolecules[31]. For an improved syntrophic relationship, the pH of the digester must be kept in the neutral range[32]. Due to its highly basic nature, biochar can relieve the acid inhibition brought about by hydrolytic acidification (Figure 1). Wang et al.[33] assessed how vermicompost biochar (VCBC) aids in acid buffering when digesting kitchen waste and chicken manure. They noted that, in comparison with the control digester without VCBC, the bioreactor with VCBC had better buffering capacity as the reduction in pH was milder. The high buffering capability of VCBC can be attributed to the high concentration of alkali-based metals (Na, K, Ca, and Mg), which are 8.47 g/kg, 2.53 g/kg, 15.82 g/kg, and 0.44 g/kg, respectively, in VCBC [34].
- Effect of biochar on microorganisms
The performance of the anaerobic digestion (AD) process relies on the collective activities of diverse functional groups of microbial communities[35]. Incorporating biochar into the AD process provides a platform for the entrapment, binding, and colonization of microorganisms, promoting the formation of biofilms and acclimatization of microbes. This facilitates interspecies electron transfer, prevents microbial washout, and enhances overall process efficiency[36] (see Figure 1). Furthermore, biochar contributes to the enrichment of beneficial microbial communities in the AD system by catalyzing their generation and activity. In addition to enriching microbial communities, the introduction of biochar can induce significant changes in the microbial community structure of the AD process, ultimately enhancing methane production. This phenomenon has been observed by several researchers. Wang et al.[37] investigated the anaerobic digestion of food waste and dewatered activated sludge with sawdust biochar. When the 16S rRNA gene sequences were analyzed, it was revealed that the application of biochar restructured the bacterial community, as it was observed that only the biochar-amended digesters had Anaerolineaceae and Methanosaeta, which are typical microorganisms involved in direct interspecies electron transfer.
Though directly applying biochar to AD improves the functionality of microorganisms, it has some limitations. One such limitation is that there is usually an alteration in the number of methane-generating microorganisms attached to biochar[38]. Additionally, it takes several days for the complex microbial communities to form on a solid support[39]. One technique that can be applied to improve this is using biochar, which is already loaded with microorganisms. To reduce costs associated with continuous production of biochar, the pre-loaded biochar can be biochar recycled from high-solids digesters or effluent from low-solids digesters[40].
- Direct Interspecies Electron Transfer
During the methanogenic stage of anaerobic digestion, hydrogenotrophic methanogens[41] play a crucial role in converting CO2 to carbonates, facilitating methane formation. This conversion heavily relies on the syntrophic relationship established between organic acid-oxidizing acetogenic bacteria and CO2-reducing methanogenic Archaea[42]. Interspecies electron transfer is essential for maintaining syntropy in the AD process, typically occurring through indirect interspecies electron transfer (IIET), where hydrogen and formate serve as electron carriers between syntrophic-producing bacteria and methanogens[43]. The exchange of metabolites between microorganisms is regulated by diffusion[44]. However, the diffusion-mediated transfer of soluble metabolites is generally slow[45], and hydrogen-mediated interspecies electron transfer poses a significant challenge to efficient methane production[46]. To enhance the rate of the digestion process, direct interspecies electron transfer (DIET) can be facilitated using conductive materials such as graphene, activated carbon, magnetite, and biochar[47]. These materials possess surface chemical properties that enable them to donate and accept electrons, thereby accelerating the conversion process. DIET has been reported by various researchers as more effective than IIET since it bypasses diffusion and is not limited by the rate of electron carrier diffusion, such as hydrogen and formate[48]. DIET has been found to expedite the conversion of diverse reduced organic compounds into methane[49]. For example, Zhao et al.[50] investigated the role of two conductive materials, magnetite, and granular activated carbon (GAC), in enhancing and stabilizing the digestion of organic waste. Magnetite was found to facilitate the breakdown of complex organics into simpler forms, while GAC promoted the syntrophic conversion of fermentation products to methane through DIET. This demonstrates that the application of activated carbon can enhance the rate of methane production in the AD process. Compared to activated carbon, biochar offers certain advantages due to its unique properties, including the presence of redox-active metal groups on its surface. For instance, Shanmugam et al.[51] studied the anaerobic digestion of glucose and aqueous phase bio-oil using biochar and GAC and observed that both materials facilitated DIET and increased methane yield. However, biochar exhibited a 72% increase in methane yield, whereas GAC increased it by 40%. The presence of redox-active compounds on the surface of biochar facilitated rapid electron movement between fermentative bacteria and methanogens (see Figure 1), contributing to its superior performance compared to other conductive materials.
- In-situ cleanup of biogas
Biochar can serve as a valuable addition to the anaerobic digestion (AD) process by facilitating the in-situ removal of carbon dioxide (CO2)[52]. Implementing biochar for on-site biogas cleanup can significantly reduce the cost of upgrading biogas to meet fuel specifications[53]. The physical properties of biochar, such as its large surface area (SSA) and high porosity, create favorable conditions for the capture of CO2 and hydrogen sulfide (H2S)[54]. Additionally, its chemical properties, including high concentrations of alkali-based metals such as potassium (K), magnesium (Mg), and sodium (Na), aid in the in-situ removal of CO2 within the bioreactor. Typically, CO2 removal involves its dissolution in water to form carbonates, followed by reactions with alkali-based metals (e.g., Ca, Mg, and K) to form calcium magnesium and potassium carbonates[55]. Biochar, with its abundant monovalent and divalent cations, acts as a catalyst for the carbonation process during AD[56] (see Figure 1). The presence of alkaline metals promotes the conversion of CO2 to carbonate/bicarbonate. Therefore, when preparing biochar for use in the AD process, it is crucial to optimize pyrolysis conditions to favor the formation of an alkaline surface that enhances in situ CO2 removal. Another approach to ensure alkaline conditions in the digester is by operating at thermophilic temperatures (50–60 °C). Digesting at elevated temperatures facilitates the release and dissolution of alkali-based metals from biochar, thereby enhancing the in-situ removal of CO2 and H2S[57]. Furthermore, compared to mesophilic conditions, thermophilic conditions result in lower toxicity levels due to improved hydrolysis and a faster microbial reaction rate, leading to the release of cations[58]. Apart from high-temperature digestion, the use of small-sized biochar particles can also enhance the availability of alkali and alkaline earth metals, promoting biochar dissolution and facilitating CO2 adsorption[59].
4. Areas of Future Research
Based on the reviewed literature, further research is needed in the following areas regarding the application of biochar in the anaerobic digestion (AD) process:
- Optimal levels of parameters for thermophilic AD:
The research suggests that conducting digestion under thermophilic conditions (between 50 and 60 °C) can mitigate volatile fatty acid (VFA) and ammonia inhibition, leading to increased biomethane yield. However, research is required to determine the optimum levels of other parameters that can be used in conjunction with thermophilic temperatures to mitigate the negative effects of high temperatures on organic waste digestion with biochar. This will help optimize the AD process and enhance its performance.
- Interdependency of different mechanisms in inhibiting AD:
In addition to thermophilic temperatures, various mechanisms, such as ammonia adsorption on biochar surfaces, have been reported to alleviate inhibition during the AD process. However, most studies have focused on investigating these mechanisms in isolation. It is essential to explore the interplay between different inhibitory factors, such as the interaction between ammonia and VFA inhibition, to gain a comprehensive understanding of their combined effects and the role of biochar in mitigating them. Dissolution may not always be the primary reason for ammonia inhibition mitigation by biochar, and further clarification is needed.
- Use of biochar-immobilized bacteria:
The research identified limitations regarding the microbial community structure in the AD process with biochar, including changes in the population of methanogenic microorganisms attached to biochar, the time required for the formation of complex microbial communities on biochar support, and fluctuations in microbial communities within the digester. Therefore, urgent research is needed on the use of bacteria immobilized on biochar or preloaded biochar. Immobilized bacteria can expedite the acclimatization process in the digester, thereby enhancing the efficiency of the AD process.
- Interactions between biochar, digestate, and soil:
Further investigation is necessary to understand the interactions between biochar, digestate (the residue from the AD process), and soil to effectively utilize the mixture of digestate and biochar as soil conditioners. Future studies should consider the agronomic value of the resulting digestate, including its nutrient content, impact on germination, and phytotoxicity index, among other relevant factors. Understanding these interactions will contribute to optimizing the use of biochar and digestate for soil improvement purposes.
5. Conclusion
Biochar offers an efficient solution for addressing multiple challenges in the anaerobic digestion (AD) process. However, certain challenges need to be addressed to maximize its effectiveness. These challenges involve making appropriate choices regarding feedstock selection, pyrolysis conditions, and biochar activation to achieve the desired product for targeted use in the AD industry. Furthermore, the dosage of biochar applied in the AD process significantly impacts its efficiency. Therefore, it is crucial to ensure that the entire process, from biochar preparation to its application in AD, promotes the development of desirable properties such as a large specific surface area (SSA), a substantial pore structure, a high pH, and surface functional groups. This research highlights that pyrolyzing biomass at high temperatures (>450 °C) facilitates the formation of these crucial properties. However, it is important to note that high-temperature pyrolysis can result in the removal of oxygen (O) and hydrogen (H) functional groups from the biochar surface. Therefore, when pyrolyzing biomass for the AD process, the choice of temperature should be based on the intended purpose of biochar in the AD system. In addition to considerations related to biomass pyrolysis, optimizing the operational parameters of anaerobic digesters is essential for achieving higher methane yield and improved biogas quality. Therefore, enhancing the design and stability of anaerobic digesters becomes necessary to maximize the benefits of biochar in the AD process.
References
- Zhang, D.; Sial, M.S.; Ahmad, N.; Filipe, A.J.; Thu, P.A.; Zia-Ud-Din, M.; Caleiro, A.B. ater Scarcity and Sustainability in an Emerging Economy: A Management Perspective for Future. . Sustainability 2020, 13, 144, 10.3390/su13010144..
- ga, M.; Muoghalu, C.; Camargo-Valero, M.A.; Evans, B.E Effect of Turning Frequency on the Survival of Fecal Indicator Microorganisms during Aerobic Composting of Fecal Sludge with Sawdust.. Int. J. Environ. Res. Public Health 2023, 20, 2668, 10.3390/ijerph20032668.
- Panigrahi, S.; Dubey, B.K. A critical review on operating parameters and strategies to improve the biogas yield from anaerobic digestion of organic fraction of municipal solid waste. . Renewable Energy 2019, 143, 779-797, 10.1016/j.renene.2019.05.040.
- Zainal, B.S.; Zinatizadeh, A.A.; Chyuan, O.H.; Mohd, N.S.; Ibrahim, S. Effects of Process, Operational and Environmental Variables on Biohydrogen Production Using Palm Oil Mill Effluent (POME). Int. J. Hydrogen Energy 2018, 43, 10637–10644, 10.1016/j.ijhydene.2017.10.167..
- Wang, D.; Ai, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Song, C. Improving anaerobic digestion of easy-acidification substrates by promoting buffering capacity using biochar derived from vermicompost. Bioresource Technology 2017, 227, 286-296, 10.1016/j.biortech.2016.12.060.
- Luo, C.; Lü, F.; Shao, L.; He, P Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Research 2015, 68, 710-718, 10.1016/j.watres.2014.10.052.
- Vavilin, V.A.; Rytov, S.V.; Lokshina, L.Y.; Pavlostathis, S.G.; Barlaz, M.A. Distributed model of solid waste anaerobic digestion: Effects of leachate recirculation and pH adjustment.. Biotechnol. Bioeng. 2003, 81, 66-73.
- Zhou, X.; Wu, S.; Wang, R.; Wu, H. Nitrogen removal in response to the varying C/N ratios in subsurface flow constructed wetland microcosms with biochar addition.. Environ Sci Pollut Res Int 2019, 26, 3382-3391, 10.1007/s11356-018-3871-4..
- Kończak, M.; Oleszczuk, P.; Różyło, K. Application of different carrying gases and ratio between sewage sludge and willow for engineered (smart) biochar production. Journal of CO2 Utilization 2019, 29, 20-28, 10.1016/j.jcou.2018.10.019.
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion.. Renewable and Sustainable Energy Reviews 2019, 103, 291-307, 10.1016/j.rser.2018.12.048.
- Kończak, M.; Oleszczuk, P.; Różyło, K. Application of different carrying gases and ratio between sewage sludge and willow for engineered (smart) biochar production.. Journal of CO2 Utilization 2019, 29, 20-28, 10.1016/j.jcou.2018.10.019.
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S.; Show, P.L.; Sawarkar, A.D.; Singh, L.; Tsang, D.C.W. A critical review on biochar for enhancing biogas production from anaerobic digestion of food waste and sludge. Journal of Cleaner Production 2021, 305, 127143, 10.1016/j.jclepro.2021.127143.
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renewable and Sustainable Energy Reviews 2016, 55, 467-481, 10.1016/j.rser.2015.10.122.
- Paritosh, K.; Vivekanand, V. Solid state anaerobic digestion of agricultural stubble for enhanced methane production. Biochar enabled syntrophic action: Solid state anaerobic digestion of agricultural stubble for enhanced methane production. 2019, 289, 121712, 10.1016/j.biortech.2019.121712.
- Indren, M.; Birzer, C.H.; Kidd, S.P.; Hall, T.; Medwell, P.R. Effects of biochar parent material and microbial pre-loading in biochar-amended high-solids anaerobic digestion. Bioresource Technology 2020, 298, 122457, 10.1016/j.biortech.2019.122457.
- Shen, Y.; Linville, J.L.; Ignacio-de Leon, P.A.A.; Schoene, R.P.; Urgun-Demirtas, M. Towards a sustainable paradigm of waste-to-energy process: Enhanced anaerobic digestion of sludge with woody biochar. Journal of Cleaner Production 2016, 135, 1054-1064, 10.1016/j.jclepro.2016.06.144.
- Paritosh, K.; Vivekanand, V. Biochar enabled syntrophic action: Solid state anaerobic digestion of agricultural stubble for enhanced methane production.. Bioresource Technology 2019, 289, 121712, 10.1016/j.biortech.2019.121712.
- Linville, J.L.; Shen, Y.; Ignacio-de Leon, P.A.; Schoene, R.P.; Urgun-Demirtas, M. In-situ biogas upgrading during anaerobic digestion of food waste amended with walnut shell biochar at bench scale. Waste Manag Res 2017, 35, 669-679, 10.1177/0734242X17704716.
- Tan, X.; Liu, Y.-g.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions.. Chemosphere 2015,, 125, 70-85..
- Pan, J.; Ma, J.; Liu, X.; Zhai, L.; Ouyang, X.; Liu, H. Effects of different types of biochar on the anaerobic digestion of chicken manure.. Bioresource Technology 2019, 275, 258-265, 10.1016/j.biortech.2018.12.068.
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. Journal of Analytical and Applied Pyrolysis 2015,, 112, 201-213,, 10.1016/j.jaap.2015.01.025.
- Abit, S.M.; Bolster, C.H.; Cai, P.; Walker, S.L. Influence of Feedstock and Pyrolysis Temperature of Biochar Amendments on Transport of Escherichia coli in Saturated and Unsaturated Soil. . Environmental Science & Technology 2012, , 46, 8097-8105, 10.1021/es300797z.
- Indren, M.; Birzer, C.H.; Kidd, S.P.; Hall, T.; Medwell, P.R. Effects of biochar parent material and microbial pre-loading in biochar-amended high-solids anaerobic digestion. Bioresource Technology 2020, 298, 122457, 10.1016/j.biortech.2019.122457.
- Cirne, D.G.; Paloumet, X.; Björnsson, L.; Alves, M.M.; Mattiasson, B. Anaerobic digestion of lipid-rich waste—Effects of lipid concentration.. Renewable Energy 2007, 32, 965-975, 10.1016/j.renene.2006.04.003.
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. . Water Research 2015,, 68, 710-718, 10.1016/j.watres.2014.10.052.
- Sossa, K.; Alarcón, M.; Aspé, E.; Urrutia, H. Effect of ammonia on the methanogenic activity of methylaminotrophic methane producing Archaea enriched biofilm.. Anaerobe 2004, 10, 13-18, 10.1016/j.anaerobe.2003.10.004.
- Koukouzas, N.; Hämäläinen, J.; Papanikolaou, D.; Tourunen, A.; Jäntti, T. Mineralogical and elemental composition of fly ash from pilot scale fluidised bed combustion of lignite, bituminous coal, wood chips and their blends.. Fuel 2007, 86, 2186-2193, 10.1016/j.fuel.2007.03.036.
- Singoredjo, L.; Kapteijn, F.; Moulijn, J.A.; Martín-Martínez, J.-M.; Boehm, H.-P. Modified activated carbons for the selective catalytic reduction of NO with NH3.. Carbon 1993, 31, 213-222, 10.1016/0008-6223(93)90175-A.
- Seredych, M.; Bandosz, T.J. Mechanism of Ammonia Retention on Graphite Oxides: Role of Surface Chemistry and Structure.. The Journal of Physical Chemistry 2007, 111, 15596-15604, 10.1021/jp0735785.
- Shen, F.; Yuan, H.; Pang, Y.; Chen, S.; Zhu, B.; Zou, D.; Liu, Y.; Ma, J.; Yu, L.; Li, X.; et al. Performances of anaerobic co-digestion of fruit & vegetable waste (FVW) and food waste (FW): Single-phase vs. two-phase.. Bioresource Technology 2013, 144, 80-85, 10.1016/j.biortech.2013.06.099.
- Cotter, P.D.; Hill, C. Surviving the Acid Test: Responses of Gram-Positive Bacteria to Low pH. . Microbiology and Molecular Biology Reviews 2003, 67, 429-453, 10.1128/mmbr.67.3.429-453.2003..
- Wang, Y.; Moe, C.L.; Null, C.; Raj, S.J.; Baker, K.K.; Robb, K.A.; Yakubu, H.; Ampofo, J.A.; Wellington, N.; Freeman, M.C.; et al. Multipathway Quantitative Assessment of Exposure to Fecal Contamination for Young Children in Low-Income Urban Environments in Accra, Ghana: The SaniPath Analytical Approach.. Am J Trop Med Hyg 2017, 97, 1009-1019, 10.4269/ajtmh.16-0408..
- Wang, Y.; Moe, C.L.; Null, C.; Raj, S.J.; Baker, K.K.; Robb, K.A.; Yakubu, H.; Ampofo, J.A.; Wellington, N.; Freeman, M.C.; et al. Multipathway Quantitative Assessment of Exposure to Fecal Contamination for Young Children in Low-Income Urban Environments in Accra, Ghana: The SaniPath Analytical Approach. Am J Trop Med Hyg 2017, 97, 1009-1019, 10.4269/ajtmh.16-0408.
- Yang, L.; Xu, F.; Ge, X.; Li, Y. Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass.. Renewable and Sustainable Energy Reviews 2015, 44, 824-834, 10.1016/j.rser.2015.01.002.
- Vanwonterghem, I.; Webster, N.S. Coral Reef Microorganisms in a Changing Climate.. iScience 2020, 23, 100972, 10.1016/j.isci.2020.100972..
- Lu, Y.; Lai, Q.; Zhang, C.; Zhao, H.; Ma, K.; Zhao, X.; Chen, H.; Liu, D.; Xing, X.-H. Characteristics of hydrogen and methane production from cornstalks by an augmented two- or three-stage anaerobic fermentation process. Bioresource Technology 2009, 100, 2889-2895, 10.1016/j.biortech.2009.01.023.
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. . Bioresource Technology 2018, 250, 812-820, 10.1016/j.biortech.2017.12.004.
- Kuroda, M.; Yuzawa, M.; Sakakibara, Y.; Okamura, M. Methanogenic bacteria adhered to solid supports. Water Research. Water Research 1988, 22, 653-656, 10.1016/0043-1354(88)90069-3.
- van Wolferen, M.; Orell, A.; Albers, S.V. Archaeal biofilm formation. Nat Rev Microbiol 2018, 16, 699-713, 10.1038/s41579-018-0058-4.
- Indren, M.; Birzer, C.H.; Kidd, S.P.; Hall, T.; Medwell, P.R. Effects of biochar parent material and microbial pre-loading in biochar-amended high-solids anaerobic digestion.. Bioresource Technology 2020, 298, 122457, 10.1016/j.biortech.2019.122457.
- Stams, A.J.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Rev Microbiol 2009, 7, 568-577, 10.1038/nrmicro2166..
- Martins, G.; Salvador, A.F.; Pereira, L.; Alves, M.M. Methane Production and Conductive Materials: A Critical Review. . Environmental Science & Technology 2018,, 52, 10241-10253, 10.1021/acs.est.8b01913.
- Martins, G.; Salvador, A.F.; Pereira, L.; Alves, M.M. ethane Production and Conductive Materials: A Critical Review. Environmental Science & Technology 2018,, 52, 10241-10253, 10.1021/acs.est.8b01913.
- Chiappero, M.; Norouzi, O.; Hu, M.; Demichelis, F.; Berruti, F.; Di Maria, F.; Mašek, O.; Fiore, S. Review of biochar role as additive in anaerobic digestion processes.. Renewable and Sustainable Energy Reviews 2020, 131, 110037, 10.1016/j.rser.2020.110037.
- Lovley, D.R. Happy together: microbial communities that hook up to swap electrons. ISME 2017, 11, 327-336, 10.1038/ismej.2016.136.
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications.. Bioresource Technology 2018, , 254, 300-311, 10.1016/j.biortech.2018.01.095.
- Chen, S.-Y.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R.J.S.R. Promoting Interspecies Electron Transfer with Biochar.. Scientific Reports 2014, 4, 12-20.
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review.. Biotechnology Advances 2014, 32, 1523-1534, 10.1016/j.biotechadv.2014.10.005.
- Barua, S.; Dhar, B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion.. Bioresource Technology 2017, 244,, 698-707, 10.1016/j.biortech.2017.08.023.
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Research 2017, 115, 266-277, 10.1016/j.watres.2017.02.067.
- Shanmugam, S.R.; Adhikari, S.; Nam, H.; Kar Sajib, S. Effect of bio-char on methane generation from glucose and aqueous phase of algae liquefaction using mixed anaerobic cultures.. Biomass and Bioenergy 2018, 108, 479-486, 10.1016/j.biombioe.2017.10.034.
- Gaga, Y.; Benmessaoud, S.; Kara, M.; Assouguem, A.; Al-Ghamdi, A.A.; Al-Hemaid, F.M.; Elshikh, M.S.; Ullah, R.; Banach, A.; Bahhou, J.; et al. New Margin-Based Biochar for Removing Hydrogen Sulfide Generated during the Anaerobic Wastewater Treatment.. Water 2022, 14, 3319., 10.3390/w14203319..
- Deng, C.; Lin, R.; Kang, X.; Wu, B.; Wall, D.M.; Murphy, J.D. What physicochemical properties of biochar facilitate interspecies electron transfer in anaerobic digestion: A case study of digestion of whiskey by-products. Fuel 2021, 306,, 121736, 10.1016/j.fuel.2021.121736.
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing pipeline-quality biomethane via anaerobic digestion of sludge amended with corn stover biochar with in-situ CO2 removal. . Applied Energy 2015, 158, 300-309, 10.1016/j.apenergy.2015.08.016.
- Mun, M.; Cho, H. Mineral Carbonation for Carbon Sequestration with Industrial Waste. Waste. Energy Procedia 2013, 37, 6999-7005, 10.1016/j.egypro.2013.06.633.
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion.. Renewable and Sustainable Energy Reviews 2019, 103, 291-307, 10.1016/j.rser.2018.12.048.
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. . Chem Soc Rev 2014, 43, 8049-8080, 10.1039/c4cs00035h..
- Shen, Y.; Forrester, S.; Koval, J.; Urgun-Demirtas, M. Yearlong semi-continuous operation of thermophilic two-stage anaerobic digesters amended with biochar for enhanced biomethane production.. Journal of Cleaner Production 2017, 167, 863-874, 10.1016/j.jclepro.2017.05.135.
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion.. Renewable and Sustainable Energy Reviews 2019, 103, 291-307, 10.1016/j.rser.2018.12.048.
