Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Nunzia Gallo.
Gelatin is a natural biopolymer that is intrinsically biocompatible and biodegradable, has low immunogenicity, and is classified as Generally Recognized as Safe (GRAS) by the United States Food and Drug Administration (FDA). It consists of an amphoteric polymer that is derived from collagen by alkaline-, acid-, or heat-based hydrolysis.
- gelatin
- drug delivery systems
- nanoparticles
- microparticles
1. Structure of Gelatin
Gelatin is a natural biopolymer that is intrinsically biocompatible and biodegradable, has low immunogenicity, and is classified as Generally Recognized as Safe (GRAS) by the United States Food and Drug Administration (FDA) [31][1]. It consists of an amphoteric polymer that is derived from collagen by alkaline-, acid-, or heat-based hydrolysis [10][2]. These treatments cause the rupture of collagen’s structural organization, leading to the loss of its conformation to varying extents. Therefore, as for collagen, the extraction sources (i.e., equine, bovine, porcine, ovine, fish) and tissues (i.e., skin, tendon, scales, bone), and the animal age, besides the applied extraction procedures, represent all the parameters influencing gelatin’s properties [32][3]. Commercially, there are two types of gelatin, Type A and Type B. Type A cationic gelatin results from the partial acid hydrolysis of collagen. During this treatment, the amide groups of glutamine and asparagine are converted into carboxyl groups, resulting in the protein isoelectric point shift to higher values (pI = 7–9) [33][4]. Type B anionic gelatin derives from the alkali-based treatment of collagen. During the alkaline hydrolysis treatment, the partial removal of the asparagine and glutamine amide groups occurs, with a consequent increase in the aspartic and glutamic acid content [34,35][5][6]. The consequent increase of the carboxyl groups makes Type B gelatin negatively charged, with a lower isoelectric point (pI = 4.7–5.5) [32,36,37,38][3][7][8][9]. Accordingly, depending on the extraction process type and parameters, gelatin can have different isoelectric points, which depend on the degree of dissociation of its free carboxyl and amino groups [32,39][3][10].
2. Properties
Generally, like collagen, gelatin is characterized by the repetition of the triplet (Gly-X-Y)n (Figure 1). One third of the chain is made up of glycine, while another third is proline or hydroxyproline [40][11]. The hydroxylation of proline and lysine residues in 4-hydroxyproline and ε-hydroxylysine, respectively, is a posttranslational modification, which is present almost exclusively in collagen [41][12]. Gelatin owns cationic and anionic groups along with hydrophobic groups approximatively in a ratio of 1:1:1. Thus, about 13% of the polypeptide chain of gelatin consists of positively charged amino acid residues (i.e., mainly lysine and arginine residues), about 12% of negatively charged amino acid residues (i.e., mainly glutamic and aspartic acid), and about 11% of hydrophobic residues (i.e., leucine, isoleucine, methionine, and valine) [40][11].
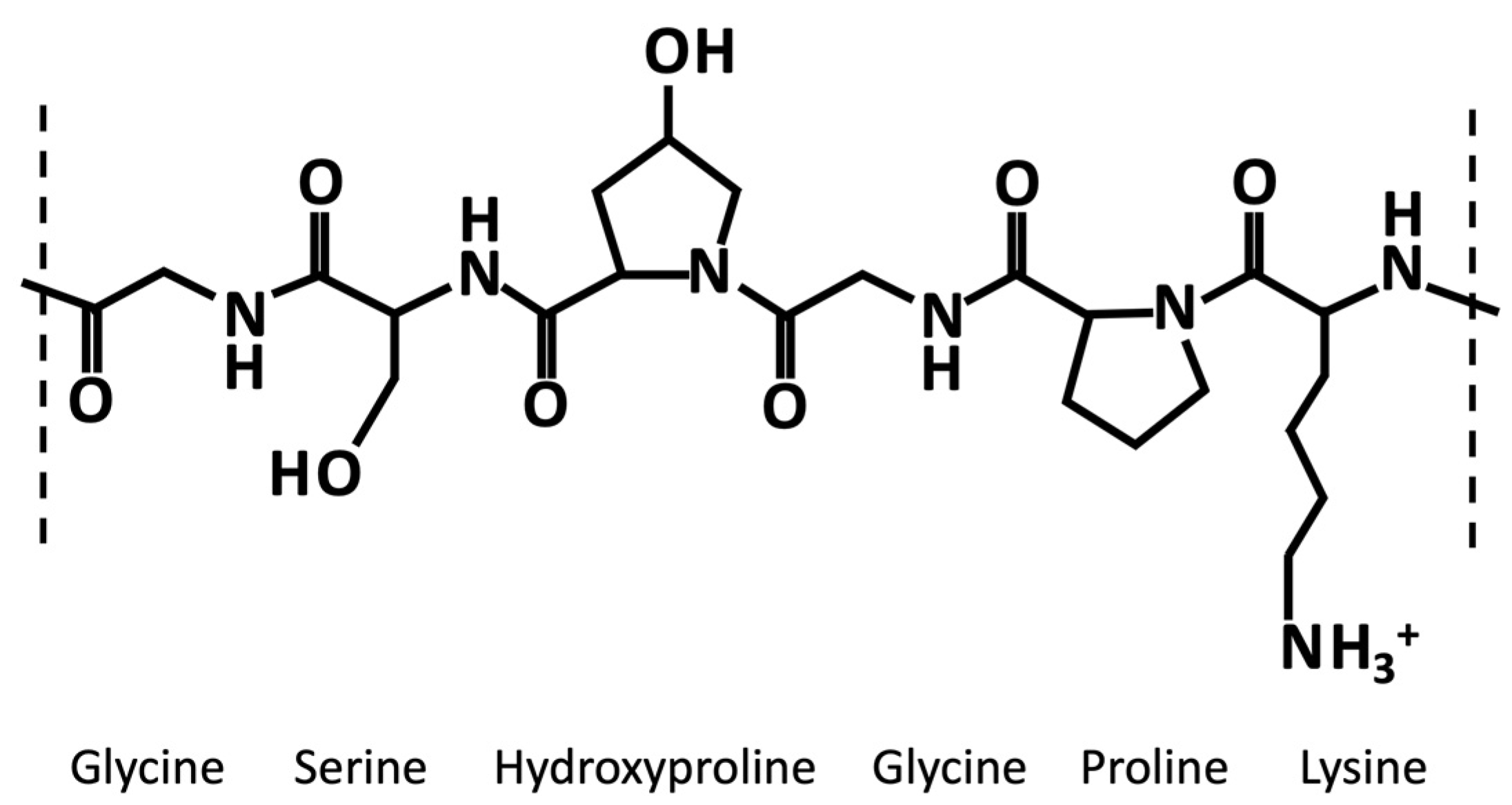
Figure 1. Exemplary chemical structure of a fragment of gelatin α-chain characterized by the repetition of the triplet (Gly-X-Y)n, where X and Y are usually proline an hydroxyproline, respectively.
The hydrolytic conversion of collagen breaks its polypeptide chains and natural structural organization. Therefore, gelatin cannot be considered as a single chemical entity with a precise molecular weight, but it rather consists of mixtures of polypeptide chains with different molecular weights that can fall in a specific range. Depending on the production process, gelatin can be characterized by different types of chains (with variable molecular weights). In particular, they could be found as (i) single α-chains of 80–125 kDa, (ii) two α-chains crosslinked in a covalent way (β-chains) of 160–250 kDa, or (iii) three covalently crosslinked α-chains (γ-chains) of 240–375 kDa (Figure 2) [39,42,43,44][10][13][14][15]. Gelatin’s protein pattern can be determined by several analytical techniques. Among them, electrophoresis and chromatography are the most-commonly performed [45][16].
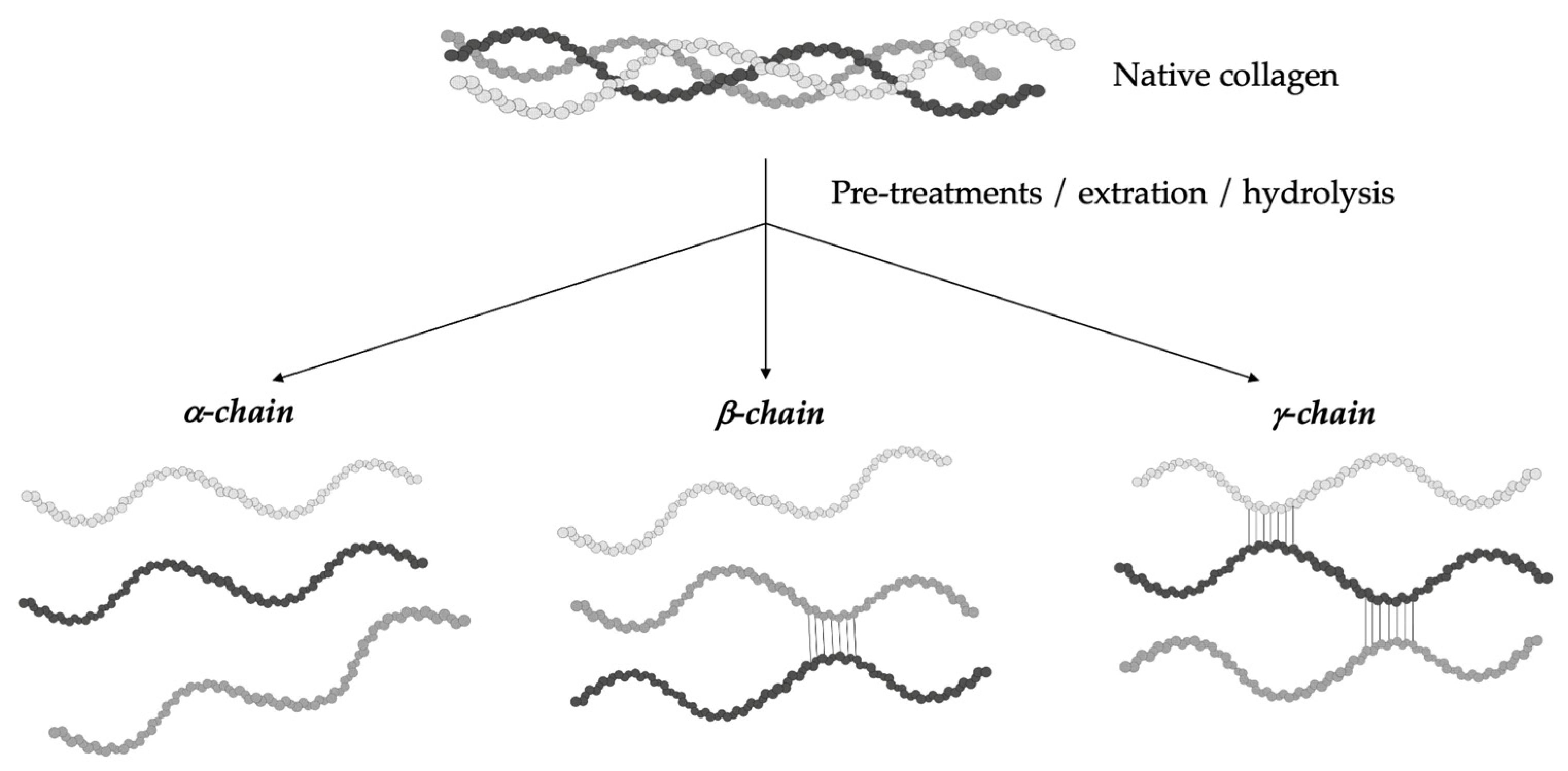
Figure 2. Gelatin is a heterogeneous mixture of water-soluble proteins with different types of chains and molecular weights depending on the production process.
Consequently, it is clear how the conformational organization of gelatin differs from that of the highly organized native collagen depending on the denaturation degree of collagen. Advanced techniques can identify the extent of collagen denaturation. Indeed, X-ray diffraction has shown a certain degree of fibril-like structures in gelatin, although these structures are not comparable to the well-organized collagen networks [39][10]. Depending on the concentration of gelatin and the temperature and the energy required for the formation of secondary structures, the polypeptide chains of gelatin may have different spatial arrangements and, thus, different interactions during gelling [46][17]. As reported by Guo et al. [47][18], three different orders of organization can be found (Figure 3). The first order is characterized by single α-chains. In the second order, α-chains can form inter-chain or intra-chain interactions, creating a loop. Lastly, the third level of order can be constituted by three different α-chains, or two α-chains, one of which creates a loop, or a single α-chain with two loops. Single-looped helices can be found in diluted solutions, while non-looped helices are more commonly found in concentrated solutions.
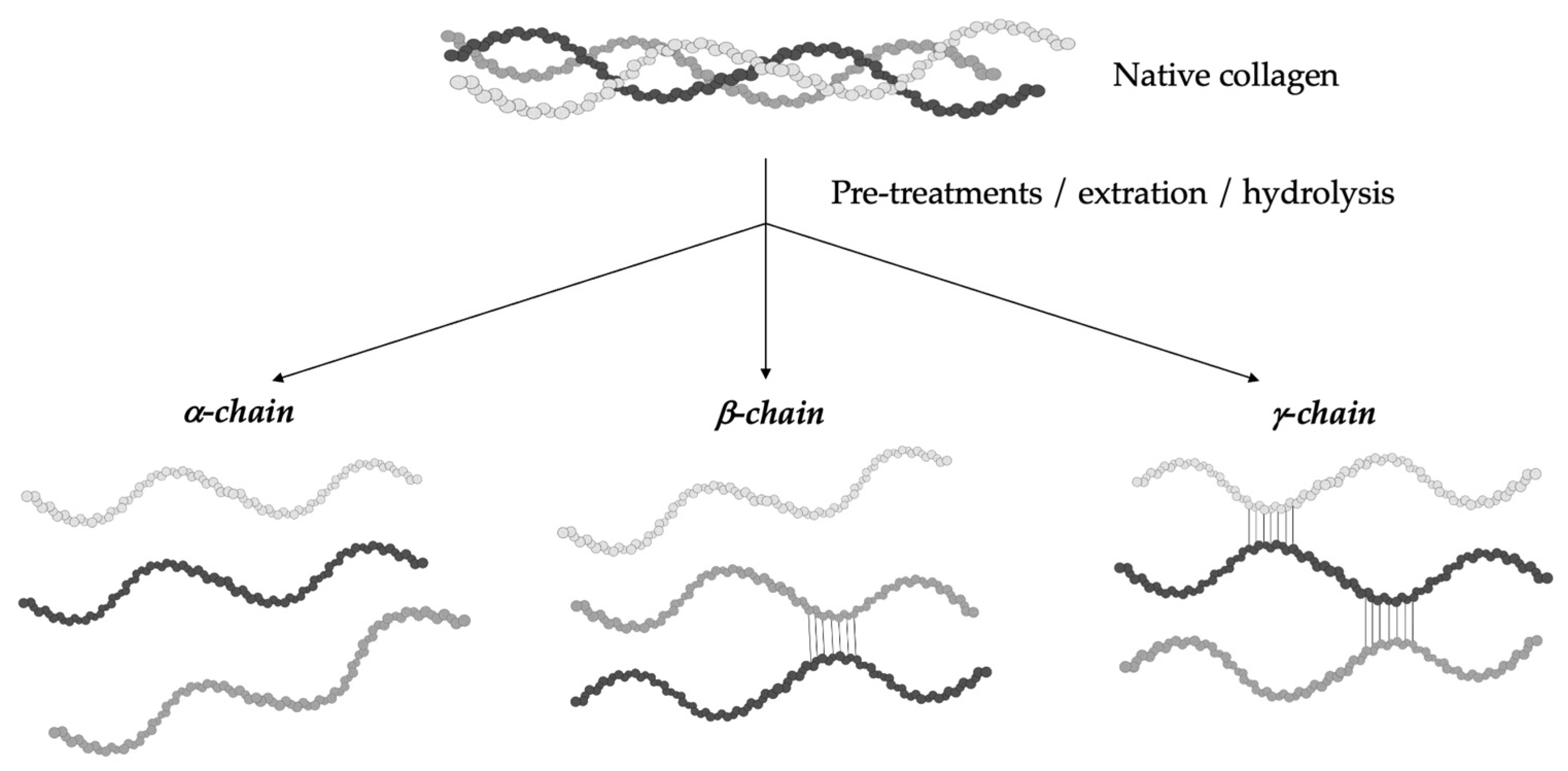
Figure 3.
The different types of gelatin chain organization.
Gelatin’s rheological properties (gel strength, viscosity) and thermal stability (melting and gelling temperature) define its quality, in addition to basic physico-chemical characteristics (solubility, composition, transparency, color, smell, and taste) [48][19]. Gel strength is defined by the so-called “Bloom value” [49[20][21][22],50,51], and it was found to decrease when the pH value was below 5 and above 9, while it remained almost constant in the pH range 5–9, with some variations [52][23]. Thus, the determination of the Bloom force is usually performed at pH values from 4.6 to 7.0 [49][20]. Another important physical property of gelatin is its viscosity, which depends on concentration, temperature, and pH. Indeed, viscosity was found to increase with polymer concentration and decrease with temperature and pH. Gelatin’s thermal stability is another important parameter that is influenced by several parameters such as polymer concentration, molecular weight distribution, and Bloom value [52][23]. However, gelatin’s melting point is usually found in the range 28–31 °C for mammal–derived gelatin and in the range of 11–28 °C for fish-derived gelatin [48,53][19][24]. Generally, the gel strength, gelling, and melting points of mammalian-derived gelatins have been revealed to be much higher than those of fish-derived gelatins. Indeed, the typical gel strength, gelling point, and melting point temperatures for mammalian gelatins are found in the range 100–300 Bloom, 20–25 °C and 28–31 °C, respectively, in comparison to fish gelatins’ values, which are about 70–270 Bloom, 8–25 °C and 11–28 °C, respectively [48,53][19][24]. As regards gelatin’s gelling time, its thermo-reversible gelation mechanism has been extensively studied. It is known that, at low temperatures, gelatin chains undergo a conformational disordered–ordered transition and are able to form thermo-reversible networks by the formation of hydrogen bonds [52,54][23][25]. In particular, gelatins are found in the sol state at high temperatures (>40 °C) as single coils. Above a determined critical concentration (usually about 1%), they are able to assemble into thermo-reversible gels with a disordered organization when the temperature is cooled down below 30 °C [55,56][26][27].
Thus, gelatin owns many advantages such as low cost, easy availability, biodegradability, and low immunogenicity, besides high biocompatibility and intrinsic bioactivity [40,57][11][28] thanks to the presence of specific arginine–glycine–aspartic (RGD) sequences, which are able to promote cell adhesion [24][29]. Moreover, being characterized by different functional groups that are easily accessible for chemical modifications (such as coupling with crosslinkers or target ligands), gelatin is widely used as a material for the manufacturing of substrates for a wide range of applications in the biomedical, pharmaceutical, cosmetic, and food sectors [58,59][30][31].
Indeed, gelatin is widely used in the food sector as a thickener (e.g., in sweets and jams), as a clarifying agent for drinks (e.g., wine, beer, fruits, and vegetables juices), an emulsifier (e.g., confectionery products), a stabilizer (e.g., ice creams, cream cheeses, and cottage cheese, as well as in food foams and salads), a texturizer, and a film former in coatings for meat and confectioneries [50,60,61,62][21][32][33][34].
As regards the cosmetic sector, gelatin is used as a gelling ingredient in various products (e.g., face creams, body lotions, shampoos, hairsprays, sunscreens, and bath salts) for its moisturizing action [63,64][35][36]. Moreover, its hydrolysates are used for nutricosmetic applications thanks to their antiaging effect [65][37].
Although gelatin seems to be mainly utilized in the food sector, actually, it is mostly used in the pharmaceutical sector as binder in the production of drugs [66][38], a stabilizer in vaccines [67][39], a material for the development of capsules and ointments [30][40], a matrix of implants and wound dressings [68][41], and for plasma expanders [69][42]. Lastly, in the biomedical field, gelatin is also used as a biomaterial for the development of DDSs and tissue engineering/regenerative medicine constructs [70][43]. In particular, gelatin has been revealed to be a good biomaterial for the manufacturing of DDSs thanks to its chemical versatility. Its high suitability in several synthesis techniques has pointed out its potential as a carrier of many types of bioactive compounds and its ability to tune and control the release kinetics of select drugs.
References
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177.
- Tan, H.; Tu, Z.; Jia, H.; Gou, X.; Ngai, T. Hierarchical Porous Protein Scaffold Templated from High Internal Phase Emulsion Costabilized by Gelatin and Gelatin Nanoparticles. Langmuir 2018, 34, 4820–4829.
- Johnston-Banks, F.A. Gelatine. In Food Gels; Elsevier Applied Food Science Series; Springer: Dordrecht, The Netherlands, 1990; Chapter 7; pp. 233–289.
- Patel, Z.S.; Yamamoto, M.; Ueda, H.; Tabata, Y.; Mikos, A.G. Biodegradable Gelatin Microparticles as Delivery Systems for the Controlled Release of Bone Morphogenetic Protein-2. Acta Biomater. 2008, 4, 1126–1138.
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic Polymers and Their Therapeutic Potential. Chem. Soc. Rev. 2012, 41, 7147–7194.
- Madkhali, O.; Mekhail, G.; Wettig, S.D. Modified Gelatin Nanoparticles for Gene Delivery. Int. J. Pharm. 2019, 554, 224–234.
- Singh, S.; Rao, R.K.V.; Venugopal, K.; Manikandan, R. Alteration in Dissolution Characteristic of Gelatin Containing Formulations: A Review of the Problem, Test Methods, and Solutions. Pharm. Technol. 2002, 26, 36–58.
- Taheri, A.; Abedian Kenari, A.M.; Gildberg, A.; Behnam, S. Extraction and Physicochemical Characterization of Greater Lizardfish (Saurida Tumbil) Skin and Bone Gelatin. J. Food Sci. 2009, 74, E160–E165.
- Zhou, P.; Regenstein, J.M. Determination of Total Protein Content in Gelatin Solutions with the Lowry or Biuret Assay. J. Food Sci. 2006, 71, C474–C479.
- Poppe, J. Gelatin. In Thickening and Gelling Agents for Food; Imeson, A.P., Ed.; Springer: New York, NY, USA, 1997; pp. 144–168.
- Elzoghby, A.O. Gelatin-Based Nanoparticles as Drug and Gene Delivery Systems: Reviewing Three Decades of Research. J. Control. Release 2013, 172, 1075–1091.
- Babel, W. Gelatine-Ein Vielseitiges Biopolymer. Tech. Chem. 1996, 30, 86–95.
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and Rheological Properties of Fish Gelatin Compared to Mammalian Gelatin. Food Hydrocoll. 2004, 18, 203–213.
- Gudipati, V. Fish Gelatin: A Versatile Ingredient for the Food and Pharmaceutical Industries. In Marine Proteins and Peptides: Biological Activities and Aplications; Wiley: Hoboken, NJ, USA, 2013; pp. 271–295.
- Duthen, S.; Rochat, C.; Kleiber, D.; Violleau, F.; Daydé, J.; Raynaud, C.; Levasseur-Garcia, C. Physicochemical characterization and study of molar mass of industrial gelatins by AsFlFFF-UV/MALS and chemometric approach. PLoS ONE 2018, 13, e0203595.
- Gomez-Guillen, M.C.; Turnay, J.; Fernandez-Diaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34.
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin Structure and Composition Linked to Hard Capsule Dissolution: A Review. Food Hydrocoll. 2015, 43, 360–376.
- Guo, L.; Colby, R.H.; Lusignan, C.P.; Whitesides, T.H. Kinetics of Triple Helix Formation in Semidilute Gelatin Solutions. Macromolecules 2003, 36, 9999–10008.
- Karim, A.A.; Bhat, R. Fish Gelatin: Properties, Challenges, and Prospects as an Alternative to Mammalian Gelatins. Food Hydrocoll. 2009, 23, 563–576.
- Wainewright, F.W. Physical Tests for Gelatin and Gelatin Products. In The Science and Technology of Gelatin; Ward, A.G., Courts, A., Eds.; Academic Press: London, UK, 1977; Chapter 16; pp. 508–557.
- Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; Wiley-VCH: Weinheim, Germany, 2007.
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827.
- Stainsby, G. The Physical Chemistry of Gelatin in Solution. In The Science and Technology of Gelatin; Ward, A.G., Courts, A., Eds.; Academic Press: London, UK, 1977; Chapter 4; pp. 109–135.
- Alfaro, A. da T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44.
- Michon, C.; Cuvelier, G.; Relkin, P.; Launay, B. Influence of Thermal History on the Stability of Gelatin Gels. Int. J. Biol. Macromol. 1997, 20, 259–264.
- Borchard, W.; Burg, B. Molecular mechanisms during the thermoreversible gelation of gelatin-water-systems. Interfaces Condens. Syst. 2007, 83, 200–210.
- Wang, R.; Hartel, R.W. Confectionery Gels: Gelling Behavior and Gel Properties of Gelatin in Concentrated Sugar Solutions. Food Hydrocoll. 2022, 124, 107132.
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2021, 42, 240–250.
- Wang, H.; Boerman, O.C.; Sariibrahimoglu, K.; Li, Y.; Jansen, J.A.; Leeuwenburgh, S.C.G. Comparison of Micro- vs. Nanostructured Colloidal Gelatin Gels for Sustained Delivery of Osteogenic Proteins: Bone Morphogenetic Protein-2 and Alkaline Phosphatase. Biomaterials 2012, 33, 8695–8703.
- Mahmoudi Saber, M. Strategies for surface modification of gelatin-based nanoparticles. Colloids Surf. B. Biointerfaces 2019, 183, 110407.
- Su, K.; Wang, C. Recent Advances in the Use of Gelatin in Biomedical Research. Biotechnol. Lett. 2015, 37, 2139–2145.
- Karim, A.A.; Bhat, R. Gelatin Alternatives for the Food Industry: Recent Developments, Challenges and Prospects. Trends Food Sci. Technol. 2008, 19, 644–656.
- Igoe, R.S. Dictionary of Food Ingredients; Van Nostrand Reinhold: New York, NY, USA, 1983.
- Clark, A.H.; Ross-Murphy, S.B. Structural and Mechanical Properties of Biopolymer Gels. Adv. Polym. Sci. 1987, 83, 57.
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The Review of Versatile Application of Collagen. Polym. Adv. Technol. 2016, 28, 4–9.
- Elgadir, M.A.; Mirghani, M.E.S.; Adam, A. Fish gelatin and its applications in selected pharmaceutical aspects as alternative source to pork gelatin. J. Food Agric. Environ. 2013, 11, 73–79.
- Fan, J.; Zhuang, Y.; Li, B. Effects of Collagen and Collagen Hydrolysate from Jellyfish Umbrella on Histological and Immunity Changes of Mice Photoaging. Nutrients 2013, 5, 223–233.
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a Delivery Vehicle for the Controlled Release of Bioactive Molecules. J. Control. Release 2005, 109, 256–274.
- Lee, E.J.; Lim, K.H. Hardly Water-Soluble Drug-Loaded Gelatin Nanoparticles Sustaining a Slow Release: Preparation by Novel Single-Step O/W/O Emulsion Accompanying Solvent Diffusion. Bioprocess. Biosyst. Eng. 2017, 40, 1701–1712.
- Zwiorek, K.; Kloeckner, J.; Wagner, E.; Coester, C. Gelatin nanoparticles as a new and simple gene delivery system. J. Pharm. Pharm. Sci. 2005, 7, 22–28.
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and Application of Gelatin as Potential Biodegradable Packaging Materials for Food Products. Int. J. Biol. Macromol. 2014, 71, 94–102.
- Weiss, A.V.; Fischer, T.; Iturri, J.; Benitez, R.; Toca-Herrera, J.L.; Schneider, M. Mechanical Properties of Gelatin Nanoparticles in Dependency of Crosslinking Time and Storage. Colloids Surf. B. Biointerfaces 2019, 175, 713–720.
- Hathout, R.M.; Omran, M.K. Gelatin-Based Particulate Systems in Ocular Drug Delivery. Pharm. Dev. Technol. 2016, 21, 379–386.
More
