Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Irshad Ahmad and Version 3 by Alfred Zheng.
Sulforaphane, derived from broccoli and other members of the Brassica genus, is a phytochemical shown to have anticancer properties. Numerous studies have shown that sulforaphane prevents the development and progression of prostatic tumors. Sulforaphane has been proposed to prevent prostate carcinogenesis by disrupting the Androgen receptor (AR) signaling pathway. Sulforaphane interacts with the promoter region of the AR gene, preventing the transcription of ARs.
- sulforaphane
- prostate cancer
- broccoli
- myrosinase
1. Introduction
The gland known as the prostate is located in the male reproductive system just behind the bladder and surrounds the urethra. The unrestricted proliferation of cells of the prostate gland results in prostate cancer [1]. Prostate carcinoma is one of the most prevalent forms of cancer in men globally, accounting for about 1.4 million new cases and 375,000 mortality per year worldwide [2]. Factors that increase the risk of developing prostate cancer include: age, genetics and lifestyle habits [3]. Considering how common prostate cancer is, the scientific community has intensified efforts in the search for novel therapeutics from naturally occurring compounds capable of preventing, inhibiting or reversing tumor development. Plants have been extensively screened for phytochemicals with anticancer properties; one such phytochemical is sulforaphane [4].
Sulforaphane is a small chemical compound found in cruciferous vegetables of the Brassica genus (broccoli, broccoli sprouts, kale, cabbage, Brussel sprouts and cauliflower). It is produced when the vegetable is chopped, chewed, boiled or disrupted, causing a plant enzyme myrosinase (EC 3.2.1.147) to convert a precursor molecule called glucoraphanin into sulforaphane.
In the 1990s, sulforaphane was isolated from broccoli for the first time and shown to possess anticancer properties by researchers at Johns Hopkins School of Medicine [5][6][6,7]. Subsequently, there has been a plethora of studies reporting the antineoplastic activity of sulforaphane. Recent studies have demonstrated that sulforaphane can prevent the development of cancer cells and initiate apoptosis in a variety of cancer types, including prostate cancer [7][8]. This is due to the compound’s ability to target multiple signaling pathways involved in cancer cell growth and survival [8][9][10][9,10,11].
2. Mechanisms of Action
2.1. AR Signaling
Androgen receptor (AR) signaling mediates the initial stages of prostate carcinogenesis [11][24]. AR is a hormone receptor and transcription factor. Binding of androgen (such as testosterone) to AR activates AR. The activated AR migrates to the nucleus where it upregulates the transcription of the genes of proteins such as B-cell lymphoma-extra-large (Bcl-XL) and Hypoxia-inducible factor (HIF-1α) [12][13][25,26]. Bcl-XL is a protein that suppresses apoptosis and thus promotes the survival and expansion of prostate cancer cells [14][27]. HIF-1α upregulates the transcription of hexokinase (HK) and pyruvate kinase (PK); over-expression of these enzymes reprograms the metabolism of cells to solely aerobic glycolysis [15][28]. This is a hallmark of cancer cells known as the Warburg effect [16][29]. Hence, HIF-1α promotes the development and multiplication of prostate cancer cells via glycolytic metabolism. Sulforaphane has been proposed to prevent prostate carcinogenesis by disrupting the AR signaling pathway. Sulforaphane interacts with the promoter region of the AR gene, preventing the transcription of ARs. This significantly reduces the synthesis of ARs, with no ARs present in the cell surface, androgens cannot bind to ARs to initiate the AR signaling cascade [17][12] (Figure 1). More so, sulforaphane has been shown to suppress HIF-1α [18][30]. Sulforaphane binds to HIF-1α and distorts its structure; the distorted HIF-1α loses its function and it is subsequently degraded (Figure 1).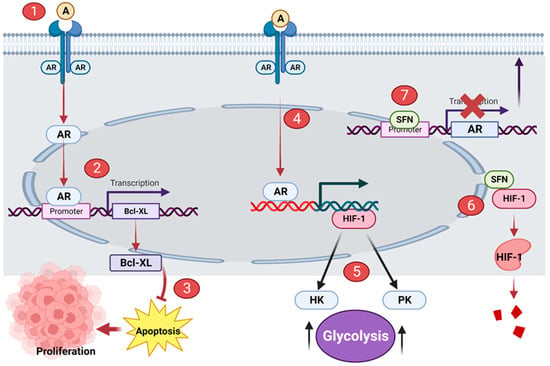
Figure 1. Sulforaphane (SFN) disrupts AR signaling in prostatic tumor cells. (1) Androgen (A) binds to AR. (2) Activated AR upregulates the transcription of Bcl-XL. (3) Bcl-XL suppresses apoptosis and promotes the proliferation of prostate cancer cells. (4) Activated AR upregulates the transcription of HIF-1. (5) HIF-1 promotes the overexpression of HK and PK; these enzymes initiate the Warburg effect. (6) SFN distorts the structure of HIF-1, the distorted protein is subsequently degraded. (7) SFN binds to the promoter region of the AR gene, interrupting its transcription and synthesis. This illustration was made with Biorender.com (accessed on 7 March 2023).
2.2. Induction of Apoptosis
Apoptosis is a natural occurrence through which the number of cells in tissues are regulated. Cancer develops when apoptosis fails; thus, cancerous tissues often suppress apoptosis in cells [19][31]. Apoptosis can be induced by the activity of the ubiquitin proteasome system (UPS). The UPS involves two processes: ubiquitination and 26S proteasome-mediated degradation. Improperly folded or damaged proteins are marked by ubiquitin, and then recognized and degraded by 26S proteasome [20][32]. The 26S proteasome has two subunits: a 20S barrel-shaped catalytic core and a 19S regulatory particle. Deubiquitinating enzymes (DUBs) are attached to the 19S regulatory particle to prevent erroneous degradation of cellular proteins. DUBs remove ubiquitin from poly-ubiquitinated protein, preventing its degradation by the proteasome [21][33]. Tumor tissues often over-express DUBs such as USP14 and UCHL5, thus preventing degradation of proteins and apoptosis, ultimately resulting in the survival and proliferation of cancerous tissues. Sulforaphane has been shown to inhibit the two proteasomal cysteine DUBs, USP14 and UCHL5, in prostate cancer cells [22][16]. Sulforaphane interacts with USP14 and UCHL5 and suppresses their activity. This promotes increased degradation and induces apoptosis of cells of prostate tumor tissue (Figure 2).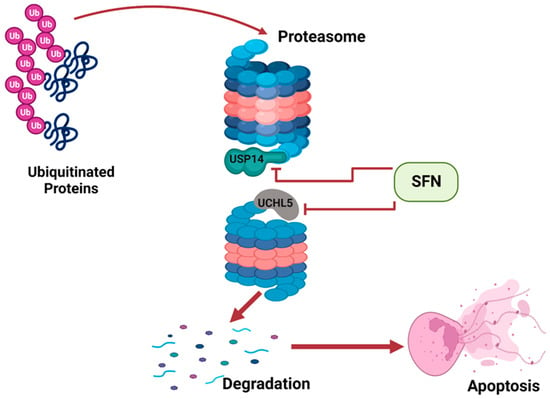
Figure 2.
SFN inhibits DUBs of the UPS to induce apoptosis. This illustration was created with Biorender.com (accessed on 7 March 2023).
2.3. DNA Damage
It has been reported that sulforaphane causes double-stranded DNA breaks and then prevents the repair of these breaks in human prostate cancer cells [23][24][14,34]. When DNA damage occurs via a double-stranded break, the repair process involves a complex of various nucleotide excision repair proteins. The combined action of MRN and CtIP proteins holds each pair of single DNA strands in place. RPA, BRCA and XPA work together to form a Holliday junction and a primer at the point of repair. Eventually, DNA synthesis is initiated and the damage is repaired [25][35]. However, in prostate cancer cells, sulforaphane inhibits XPA protein, an important protein involved in nucleotide excision repair. This disrupts and prevents the repair process; multiple double-stranded DNA breaks accumulate in the cell until the cell is destroyed by apoptosis [24][34] (Figure 3).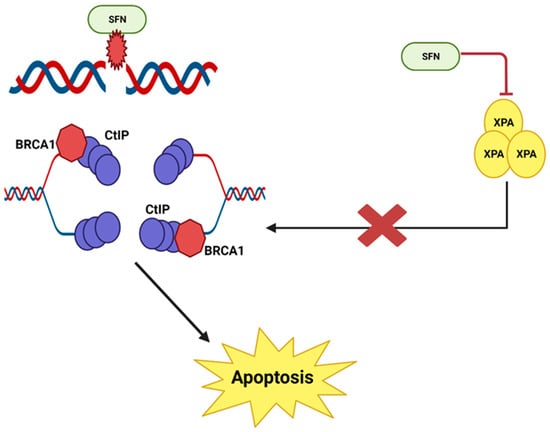
Figure 3.
SFN causes DNA damage and prevents DNA repair in prostate cancer cells. This illustration was made with Biorender.com (accessed on 7 March 2023).
2.4. Upregulation of Protective Enzymes
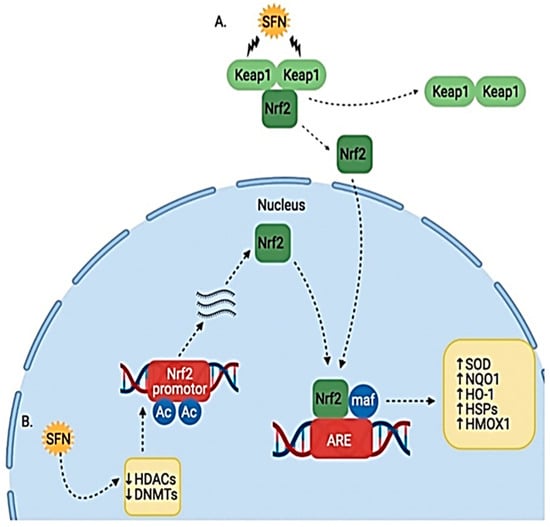
Sulforaphane protects against prostate carcinogenesis by upregulating the transcription of carcinogen-detoxifying enzymes (Phase 2 enzymes). Sulforaphane binds to Keap1 in the cytoplasm and disrupts its orientation. This disruption releases Nuclear factor erythroid 2 (Nrf2). Nrf2 is transported to the nucleus, where it binds to antioxidant response element (ARE); this leads to the increased transcription of Phase 2 detoxifying enzymes such as quinone 1, NAD(P)H dehydrogenase and heme oxygenase 1. These enzymes enhance cellular defenses and prevent the initiation of carcinogenesis [26][27][5,36] (Figure 4).