The skin plays an important role in the maintenance of the human's body physiological homeostasis. It acts as a coverage that protects against infective microorganism or biomechanical impacts. Skin is also implied in thermal regulation and fluid balance. However, skin can suffer several damages that impede normal wound-healing responses and lead to chronic wounds. Since the use of autografts, allografts, and xenografts present source limitations and intense rejection associated problems, bioengineered artificial skin substitutes (BASS) have emerged as a promising solution to address these problems. The advances that have been produced on tissue engineering techniques have enabled improving and developing new arising skin substitutes. Despite this, currently available skin substitutes have many drawbacks, and an ideal skin substitute has not been developed yet. The translation of cell‐based arising skin substitutes to clinical application represents one of the critical challenges on tissue engineering and it has to be overcome with the aim of offering each patient the more efficient therapy that fits with his clinical case and allows him having a good quality of life.
The skin plays an important role in the maintenance of the human’s body physiological homeostasis. It acts as a coverage that protects against infective microorganism or biomechanical impacts. Skin is also implied in thermal regulation and fluid balance. However, skin can suffer several damages that impede normal wound-healing responses and lead to chronic wounds. Since the use of autografts, allografts, and xenografts present source limitations and intense rejection associated problems, bioengineered artificial skin substitutes (BASS) have emerged as a promising solution to address these problems. The advances that have been produced on tissue engineering techniques have enabled improving and developing new arising skin substitutes. Despite this, currently available skin substitutes have many drawbacks, and an ideal skin substitute has not been developed yet. The translation of cell‐based arising skin substitutes to clinical application represents one of the critical challenges on tissue engineering and it has to be overcome with the aim of offering each patient the more efficient therapy that fits with his clinical case and allows him having a good quality of life.
- Dermatology
- Wound healing
- Skin
- Tissue egineering
- Advanced therapy
- Biomedicine regenerative
1. Introduction
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
Definition
The skin plays an important role in the maintenance of the human’s body physiological homeostasis. It acts as a coverage that protects against infective microorganism or biomechanical impacts. Skin is also implied in thermal regulation and fluid balance. However, skin can suffer several damages that impede normal wound-healing responses and lead to chronic wounds. Since the use of autografts, allografts, and xenografts present source limitations and intense rejection associated problems, bioengineered artificial skin substitutes (BASS) have emerged as a promising solution to address these problems. Despite this, currently available skin substitutes have many drawbacks, and an ideal skin substitute has not been developed yet. The advances that have been produced on tissue engineering techniques have enabled iimproving and developing new arising skin substitutes.
1. Introduction
Skin is the greatest organ of the human anatomy. It constitutes a protective barrier that isolates our body from harmful agents and injuries. The skin structure consists of three differentiated layers, which are the epidermis, dermis, and hypodermis. Each one has its own characteristics such as physical properties or cell composition. Epidermis is the most superficial layer, and it is composed principally of keratinocytes. Dermis, in the middle, is comprised mainly of fibroblasts, while it also has a considerable quantity of endothelial cells (ECs) that form dermal vascular networks. On the hypodermis, the inner layer, adipocytes, and EC can be found.
Our skin has the ability to self-renew. However, it can suffer several damages such as burns or deep wounds that cannot regenerate on their own. In addition, several skin injuries are associated with large and broad clinical problems that can end in morbidities. Around more than 11 million people are affected by burn injuries worldwide, and the prevalence of infection in burn units is 66% [1].
Skin grafts have been used for a long time as a solution to cover wounds; however, it is not even possible to dress massive wounds with grafts. The location of the injury, the patient state, the difficult of having a healthy donor site, and multiple factors make this approach impossible. So, there has been a real need to research another efficient way to treat these patients.
Tissue engineering [2][3][2,3] is an emergent field that is constantly evolving to provide clinical solutions for patients who need tissue replacement. A large amount of tissues has been recreated under laboratory conditions, coming to create complete organs. However, the first tissue-engineered organ that went from preclinical research to clinical application was the skin [4].
Skin bioengineered substitutes emerged as new advanced therapies to minimize all the problems that are associated with skin transplantation. These substitutes are developed in vitro to recreate human skin. Apart from being employed as grafts for the renewal of missing skin, they can be used also for other applications such as in vitro human skin models development for diseases or for testing pharmaceutical products.
Many skin substitutes for wound cover are commercially available, while a large amount of them are at the preclinical research stage. They vary on multiple properties such as the number of layers, composition, cost, technique used for its fabrication, etc. Despite the amount of skin substitutes that can be found, they have similar purposes: providing the patient with a barrier, serving as a protection against microorganisms, reducing pain, and promoting wound healing. This last topic is in the point of view of many tissue engineering researchers. Note that the goal is not only covering the wound; a skin substitute that enhances the healing process to provide wellness to the patient has to be created. Obtaining a full-layer vascularized skin substitute could accelerate wound heal and ameliorate the quality of life of burn patients. For example, children and adolescents are more susceptible to hypertrophic scarring during growth [5], so a vascularized skin substitute could be a suitable approach for a complete and effective wound heal. In addition, healing capability is affected by aging, because of a decrease in skin elasticity and strength and a reduction in blood flow to the extremities [6], so a prevascularized substitute could mitigate the healing process, especially in these cases.
2. Chronological Review
2. Chronological Review
The original report of using skin grafts dates back to 2500 BC, when they were used in India to treat serious injuries located on the extremities [7][8]. It was called the "“Ancient Indian Method"”. However, up to the beginning of the nineteenth century, nothing of importance was done in regard to skin grafts. The controversy that sparked Reverdin in 1869 with the report that he presented to the Société Impériale de Chirurgie in Paris, about the healing of granulating wounds with small bits of skin that he called "“epidermic grafts"”, and its discussion, started the flame of interest in the subject. Finally, he admitted that his graft had a portion of corium, so it was not an epidermic graft.
In the 19th century, skin grafting experienced an important technological evolution. Xenografts were developed in 1804, and the use of allografts instead of autologous skin grafts emerged in the 1870s with Thiersch. His method of taking large films of epidermis with a thin portion of dermis which was presented at the Fifteenth Congress of the German Surgical Association in 1886. Ollier did the same thing 12 years previously, but Thiersch gave no credit to him when he presented his method [7][8].
Although the use of autologous skin grafts has numerous advantages, they were not able to replace substantial skin loss, and allogenic skin grafts carried a risk of rejection. Other alternatives were emerging in response to these drawbacks.
Synthetic grafts were first described in 1880, and Mangoldt invented the "“epithelial cell seeding"” concept in 1895 [8][9]. It was the beginning of a new research area. When Rheinwald and Green cultured epidermis from a patient'’s own cells in 1975 [9][10], they did not know that they were setting a precedent in the tissue engineering field [4]. It was a revolutionary milestone and it has fostered intense research in this area since then. In 1981, O´Conner et al. created autologous sheets of cultured epithelium from keratinocytes cell expansion from two burn patients. These epithelial sheets were used to cover their extensive full thickness, becoming the first grafting of autologous epithelium on extensive burn patients with success around the world [10][11][11,12].
These cultured epidermal autografts (CEAs) were also used with success on two pediatric patients [12][13]. Unfortunately, a sheet conformed only by keratinocytes is very fragile, and it is not always possible to use it in the clinical practice.
Burke et al. generated a dermal substitute in 1981, and they used it to treat ten patients with extensive burn injuries [13][14]. That artificial dermis was used on major burns by Heimbach et al., in a randomized multicentric clinical trial, and it is currently known as an Integra™ Dermal Regeneration Template. Cuono et al., by the mid-1980s, demonstrated the importance of having a dermal vascularized component as a bed for CEA having his own method for its preparation [14][15]. The 1990s was the decade where composite skin substitutes appeared, starting from Cuono'’s technique to integrate both a dermal and an epidermal layer in one substitute. Several research centers with fairly reproducible success adopted his strategy since that decade [15][16][16,17].
These events caused the birth of what Langer and Vacanti described in 1993 [2] with the term "“tissue engineering"”. Since then, a large range of skin substitutes have been manufactured. From single-layer substitutes to artificial skins or genetically modified substitutes and so on can be found. Each substitute has its own singularity, and today´s research in this field is focusing on the in vitro creation of personalized substitutes that avoid the risk of rejection and allow the transplantation of any surface needed without limitations.
Figure 1 shows the evolutive timeline of the events described above.
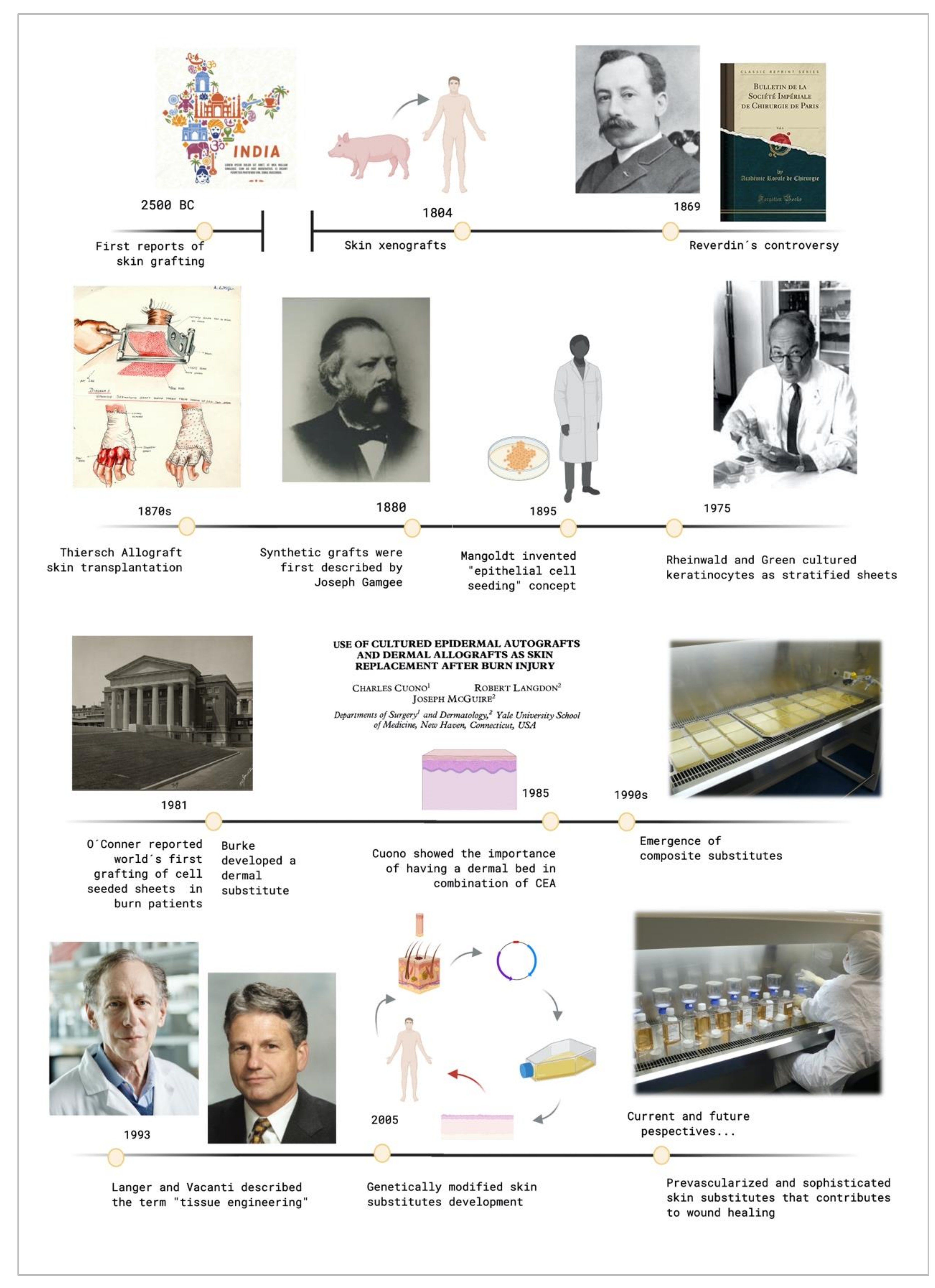
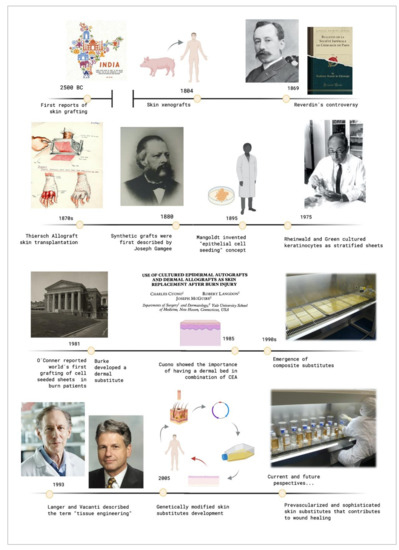
Figure 1. Timeline of the crucial events that contributed to tissue engineering development. Created with BioRender.com.
3. Clinical Demands for Bioengineered Artificial Skin Substitutes
Bioengineered skin equivalents offer promising perspectives. However, they have to fulfill a series of characteristics that allows its clinical use. As being a cell-therapy, they encounter some limitations as they are considered "Advanced Therapy Medicinal Products" (ATMPs), which applies the same good manufacturing practice (GMP) procedures as for pharmaceutical products and biological drug development [17].
However, in cases where the patient state is very serious, and other therapies have not made any effect, hospital exemptions could be needed for "compassionate use" of the skin substitutes to assure a patient's safety.
Skin substitutes must be sterile and able to protect the wound from infectious agents and the cells included on them should be capable of proliferating and differentiating in a similar manner as it happens on their natural environment, in addition to having proper functions. They have to transmit water in a similar way to the normal skin, avoiding water loss or wateraccumulation [18]. Skin substitutes have to conform irregular surfaces and withstand shear forces and mechanical tensions that can be applied during the application of the skin equivalent to the patient. They should be made of
a combination of at least dermal and epidermal components to make an effective skin substitute [19][20][21].
4. Skin Substitutes Classification
Skin substitutes classification depends on the issue in focus. As many factors have to be taken into account, skin substitutes classification has been conflicting and overlapping in many cases. Figure 2 shows the principal parameters that are used to classify skin substitutes.
3. Clinical Demands for Bioengineered Artificial Skin Substitutes
Bioengineered skin equivalents offer promising perspectives. However, they have to fulfill a series of characteristics that allows its clinical use. As being a cell-therapy, they encounter some limitations as they are considered “Advanced Therapy Medicinal Products” (ATMPs), which applies the same good manufacturing practice (GMP) procedures as for pharmaceutical products and biological drug development [18]
However, in cases where the patient state is very serious, and other therapies have not made any effect, hospital exemptions could be needed for “compassionate use” of the skin substitutes to assure a patient ́s safety.
Skin substitutes must be sterile and able to protect the wound from infectious agents and the cells included on them should be capable of proliferating and differentiating in a similar manner as it happens on their natural environment, in addition to having proper functions. They have to transmit water in a similar way to the normal skin, avoiding water loss or water accumulation [28]. Skin substitutes have to conform irregular surfaces and withstand shear forces and mechanical tensions that can be applied during the application of the skin equivalent to the patient. They should be made of a combination of at least dermal and epidermal components to make an effective skin substitute [32–34]
4. Skin Substitutes Classification
Skin substitutes classification depends on the issue in focus. As many factors have to be taken into account, skin substitutes classification has been conflicting and overlapping in many cases. Figure 2 shows the principal parameters that are used to classify skin substitutes.
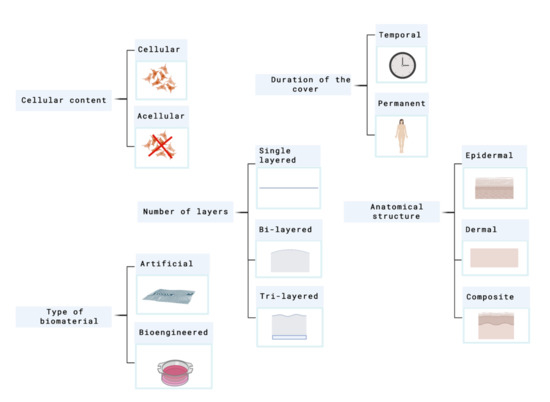
Figure 2.
In 2001, Balasubramani and their colleagues [22][36], proposed a classification where they put the skin substitutes into three categories attending to the next criteria:
-
I. Cultured epidermal substitutes
I. Cultured epidermal substitutes
-
II. Dermal components coming from skin or extracellular matrix (ECM) components
II. Dermal components coming from skin or extracellular matrix (ECM) components
-
III. Substitutes that include both dermal and epidermal components.
III. Substitutes that include both dermal and epidermal components.
This system does not differentiate between cellular and acellular components and does not include dermal constructs fabricated from synthetic polymers or dermal substitutes such as Integra®.
Balasubramani'´s classification was replaced by Kumar'´s system, which was published in 2008. Currently, it is considered the most frequently used classification system in this field [23][37].
Figure 3 shows this classification.
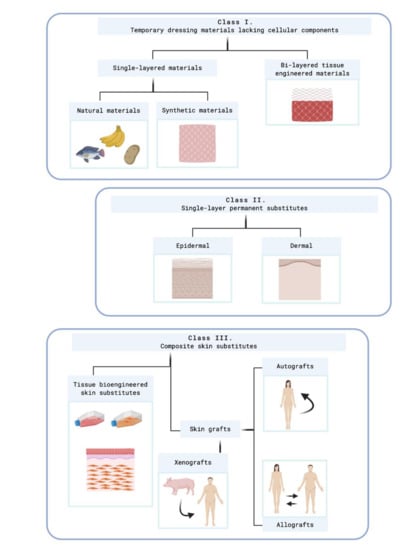
Figure 3. Kumar's classification system. Created with BioRender.com. At class II, subclass epidermal, Kumar includes cultured epidermal autografts (CEA) and Apligraf
®
®
®
®
®
However, it is necessary to highlight that a standard classification system does not exist. The parameters showed above have similar importance so, finally, each author could classify the skin equivalents under its criteria.
4.1. Temporary Skin Substitutes
4.1. Temporary Skin Substitutes
Temporary skin substitutes play a crucial role in the process of wound healing because they allow skin regeneration while the permanent skin substitute is not implanted yet. They are used as a first dress on deep injuries. When the wound is covered, infection risks are reduced, which indirectly also reduces corrective surgeries and hospitalization costs [17][18].
Conventional temporary skin substitutes are xenogeneic decellularized skin and allogenic cadaveric human skin. Porcine xenografts are probably the most frequently used temporary skin covering worldwide, which is attributable to its easy accessibility and storage [24][38]. Allogenic skin equivalents that comes from human cadaveric skin become engrafted to the wound site between 14 and 21 days, and when it is taken out at surgery, it leaves the dermal components as a viable bed for CEA [16][17]. However, the main drawbacks of conventional substitutes are rejection-related risks. Other temporary skin substitutes are synthetic substitutes, human amniotic membrane, and other natural skins. Table 1 summarizes different types of temporary skin substitutes.
Table 1. Classification of temporary skin substitutes 1.
|
Type of Temporary Cover |
Example |
Description |
|
Xenogeneic decellularized skin |
E-Z Derm® Mölnlycke |
Porcine xenograft for skin loss injuries |
|
Allogenic cadaveric human skin |
Euro Skin Bank |
Donated human skin allografts derived from cadavers |
|
Human amnion |
EpiBurn® Mimedx |
Dehydrated human amnion allograft which acts as a protective barrier and promotes healing |
|
Synthetic dressings |
Gauzes or hydrocolloids |
Covers made of synthetic materials |
|
Alternative natural skin covers |
Banana leaves Potato peel |
Natural covers used specially on developing countries |
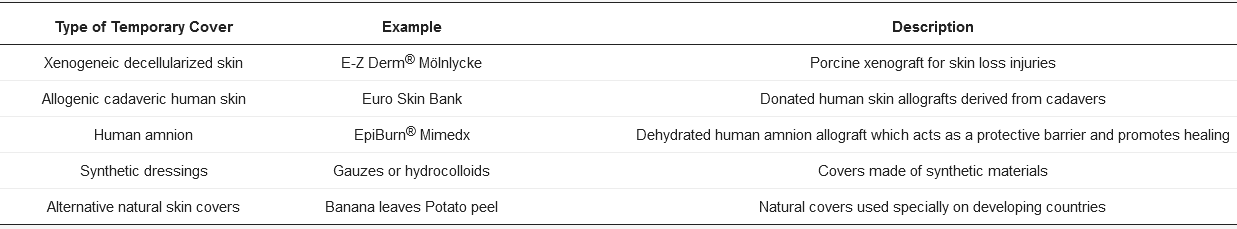
1 Types of temporary covers: examples and description.
Synthetic substitutes are principally composed of hydrogels and hydrofibers [25][39]. Despite these synthetic dressings acting as a cover capable of protecting skin injury, they do not contribute to skin tissue regeneration, especially in the case of patients who present extensive wounds.
The human amniotic membrane (HAM), the innermost layer in the fetal placenta, is another element that has been used as a successful biological skin substitute in wounds. It has been shown that it accelerates wound healing, prevents infection, and alleviates pain [26][40]. HAM is a special tissue with anti-inflammatory and anti-fibrotic properties [27][41]. Amniotic epithelial cells (AECs) and amniotic mesenchymal cells (AMCs) compose the HAM. Both kind of cells have self-renewal properties and the capability to differentiate to multiple lineage [28][42].
Other alternative natural skins such as banana leaves [29][43], potato peel [26][40], or tilapia fish skin [30][44] are used as first covers in developing countries where more sophisticated therapies are off-limits to the population.
As temporary substitutes are "“temporal solutions"”, a permanent skin substitute must dress the injury to replace the missed skin. The category of permanent skin substitutes includes the grafting of cultured skin cells with or without the support of a scaffold. There are many skin substitutes that are still in the research stage; however, a broad range of commercial skin substitutes can be encountered on the market.
5. Commercially Available Skin Substitutes
Skin substitutes up until now majorly focused on designing natural, artificial, or mixed dermal and epidermal components. In case of full thickness wound healing, all the three layers of skin need to be regenerated, which can occasionally include muscular tissue as well [31]. However, a commercially available skin substitute whose structure mimics the human rheology of the three skin layers, epidermis, dermis, and hypodermis, has not been already fabricated. Even more, a prevascularized skin equivalent on the market have not be found. Current available skin substitutes lack a layer that simulates hypodermis, considering the importance of this layer. It can be intuited that full‐layer skin substitutes that contain a hypodermal layer are not yet well established in the panorama of tissue engineering. This kind of skin substitute will be useful specially in the case of deep wounds where the subcutaneous layer is lost. New arising skin substitutes may include an hypodermic vascularized layer to provide a better integration with the host tissue and an early wound heal due to a rapid blood supply to the construct.
5. Commercially Available Skin Substitutes
Skin substitutes up until now majorly focused on designing natural, artificial, or mixed dermal and epidermal components. In case of full thickness wound healing, all the three layers of skin need to be regenerated, which can occasionally include muscular tissue as well [83]. However, a commercially available skin substitute whose structure mimics the human rheology of the three skin layers, epidermis, dermis, and hypodermis, has not been already fabricated. Even more, a prevascularized skin equivalent on the market have not be found. Current available skin substitutes lack a layer that simulates hypodermis, considering the importance of this layer. It can be intuited that full‐layer skin substitutes that contain a hypodermal layer are not yet well established in the panorama of tissue engineering. This kind of skin substitute will be useful specially in the case of deep wounds where the subcutaneous layer is lost. New arising skin substitutes may include an hypodermic vascularized layer to provide a better integration with the host tissue and an early wound heal due to a rapid blood supply to the construct.
