The ubiquitin–proteasome system (UPS) is an essential part of the cellular machinery responsible for maintaining intracellular protein homeostasis. A network of proteins that comprises the proteolytic system and chaperones calculates cellular protein homeostasis. Chaperones are in charge of correcting protein misfolding, but the proteolytic system, which converges on the 26S proteasome, is in charge of removing damaged or unfolded proteins to maintain a healthy environment inside the cell. Using proteolysis-targeting chimera (PROTAC) technology for targeted protein degradation, a novel technique of treatment is emerging that stems from an aberrant expression of a protein that causes disease. PROTAC molecules are tiny, bifunctional molecules that bind an E3-ubiquitin ligase and a target protein at the same time, causing ubiquitination and proteasome destruction of the target protein.
- PROTACs
- proteolysis
- emerging medicine
- degradation
- druggable technologies
1. Background, Composition, and Mechanism of Function
2. Proteolysis-Targeting Chimeras: Targeted Management Strategy for Several Diseased Conditions
2.1. Proteolysis-Targeting Chimeras in the Cancer Treatment
2.2. Proteolysis-Targeting Chimeras in Immune System Diseases
2.3. Proteolysis-Targeting Chimeras in Viral Infection
2.4. Proteolysis-Targeting Chimeras in Metabolic Disease
3. Therapeutic Application of Proteolysis-Targeting Chimera
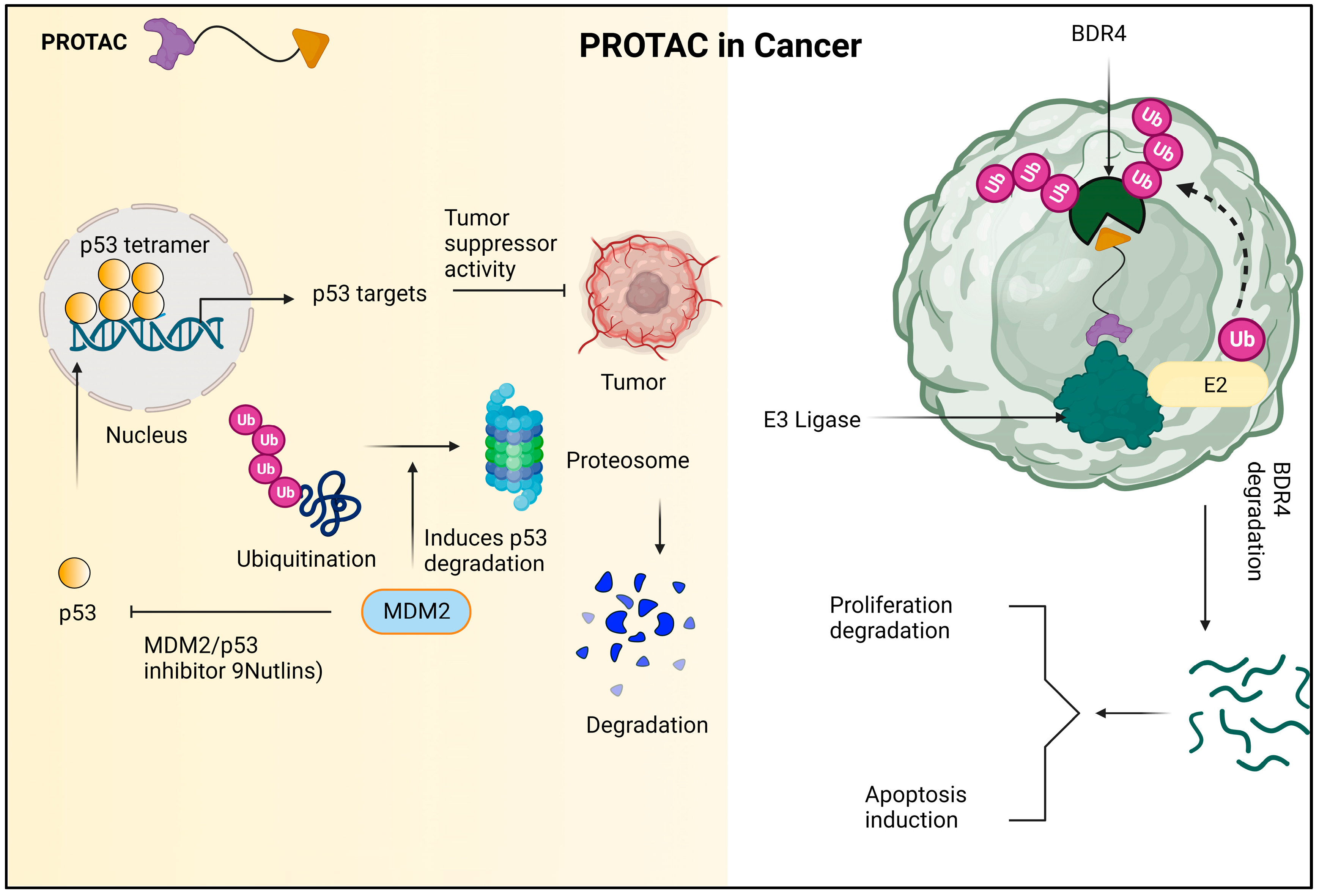
Proteolysis-targeting chimeras (PROTACs) have fundamentally altered the way that medications are created due to their numerous advantages over traditional small-molecule inhibitors. As opposed to occupancy-driven inhibitors, PROTACs’ mechanism of action (MOA) is event-driven and catalytic in nature, leading to a stronger and longer-lasting effect. Furthermore, PROTACs offer a further level of selectivity that reduces potential toxicity and boosts effectiveness in the face of drug-resistance mechanisms. By concentrating on non-enzymatic processes, they can increase the range of potential treatment targets. Since its discovery twenty years ago, PROTACs have developed from cell-impermeable peptide–small-molecule hybrids to clinical candidates that are orally bioavailable and can break down oncogenic proteins in people. The pace of scientific advancement is expected to quicken as researchers approach the third decade of targeted protein degradation (TPD). The creation of ligands for previously “undruggable” proteins and the recruitment of new E3 ligases are made possible by advances in technology. Furthermore, improved computational power is assisting in the logical design of more powerful and selective PROTACs as well as the discovery of active degraders [33]. When opposed to conventional inhibitors, PROTACs, also known as proteolysis-targeting chimeras, offer a novel pharmacodynamic strategy with a number of potential advantages. The ability to achieve pharmacodynamic efficacy even when the PROTACs are not detectable in the body is one of these benefits [34]. Selectivity, effective distribution, and sensitivity to drug resistance are just a few of the advantageous characteristics of PROTACs. These features can be enhanced by using targeting ligand methods. These compounds function by selectively stimulating intracellular proteolysis, which has been shown to be effective in inhibiting cancer cell proliferation and encouraging apoptosis [35]. A particular class of molecule known as PROTAC can benefit from the advantageous interaction between two amyloid proteins. The PROTAC-induced close proximity of the amyloids allows for the formation of a stable ternary complex, which can boost the cross-interaction’s beneficial effects. The PROTAC molecules will be built using peptide mimics with a high affinity for a 1-42 [36][37]. Alzheimer’s disease and related tauopathies are characterized by tau buildup within cells, and targeting tau has emerged as a promising strategy for therapeutic development. In order to selectively degrade proteins inside of cells, the proteolysis-targeting chimera (PROTAC) approach was developed. In order to achieve this, a novel small-molecule PROTAC with the designation C004019 and a molecular mass of 1035.29 dalton was developed. PROTAC was created to specifically increase tau protein ubiquitination and proteolysis by recruiting tau and E3-ligase (VHL) [38]. The first VHL-based small-molecule PROTAC designed to target nuclear hormone receptors was called PROTAC-ERR. These PROTACs have a lot of medicinal promise [39]. A substance called PROTAC-ERR can aim to degrade estrogen-related receptor alpha. The orphan receptor estrogen-related receptor alpha (ERR), which is found in the nucleus of MCF-7 breast cancer cells, can be targeted for degradation by the substance PROTAC-ERR. At a dose of 100 nM, PROTAC-ERR can cause these cells to degrade approximately 50% of ERR. The tyrosine kinase family of enzymes, which JAKs are a member of, is involved in the transmission of cytokine-mediated signals in cells. JAKs phosphorylate tyrosine residues when they are activated, which can subsequently activate downstream signaling proteins and cause a variety of physiological effects. These enzymes are able to transmit information from external chemicals such as cytokines, growth factors, and chemokines to the cell’s nucleus, where they can directly affect DNA transcription and the translation of a number of proteins. The highlighted paragraph discusses how various JAK proteins express themselves differently in various cell types. The widespread expression of JAK1, JAK2, and TYK2 contrasts with the predominant presence of JAK3 in hematopoietic, myeloid, and lymphoid cells. The patent also lists a number of JAK2-binding PROTAC substances, such as ruxolitinib and baricitinib, which work by targeting JAK2 JH1 in people. In MHHCALL-4 cells, the substances were examined for their capacity to cause protein degradation, cytotoxicity, and effects on the JAK-STAT signaling pathway [40]. Protein kinases—which are often mutated in the human genome and involved in cellular processes such as cell apoptosis, signaling, signal transduction, and immune-response propagation—are linked to cancers. Both IL-1R and TLR dimerize as a result of ligand binding, and adaptor molecules are recruited to a highly conserved toll/IL-1R (TIR) domain on the cytoplasm. E3 ubiquitin ligases have received increased attention recently in drug development attempts because they are more desirable therapeutic targets. MDM2 inhibitors, which target the E3 ligase mouse double minute 2 homologue (MDM2), and von Hippel–Lindau (VHL) tumor suppressor, which is the substrate recognition component of the E3 ligase complex VCB, are two examples of specific ligands that have been developed to bind to these ligases. By responding to DNA damage or stress and controlling cell development, the tumor suppressor gene p53 also exerts a significant influence on apoptosis [41]. A possible method for causing tailored protein degradation is the proteolysis-targeting chimera (PROTAC) technology. In this strategy, heterobifunctional molecules are used to attract an E3 ubiquitin ligase to a particular protein of interest, causing the proteasome to degrade it. Indirectly affecting upstream signaling cascades, transcriptional programs, or epigenetic processes can result in PROTAC-mediated protein degradation. This technology has proven to be successful in numerous preclinical and clinical investigations, demonstrating its potential as a cutting-edge treatment strategy [42]. A promising small-molecule therapy approach for treating disorders connected to the androgen receptor (AR), such as prostate cancer, Kennedy’s disease, and cardiovascular conditions, is the PROTAC idea. The capacity of PROTACs to lower protein levels is one of its main benefits. When compared to conventional small-molecule inhibitors, this property allows for small-molecule degraders to achieve a more comprehensive target inhibition, which could lead to more effective treatments [43]. A prospective target for the therapy of numerous disorders, including cancer, is cyclin-dependent kinases (CDKs). Compound iCDK9, a highly selective CDK9 inhibitor, is one possibility. The possible toxicity of this inhibitor and incomplete understanding of its mechanism, however, pose some limits. A class of bioactive molecules known as PROTAC (proteolysis-targeting chimeras) degraders can selectively stimulate the degradation of their target protein in vitro and in vivo, therefore lowering the dose-limiting toxicity of small-molecule medications [44]. Ao et al. (2023) recently developed and synthesized [44] bifunctional PROTAC compounds to target iCDK9 and show its hitherto unidentified target and pharmacological mechanism. The CD-5 chemical showed minimal toxicity in cells while selectively degrading CDK9. While CRBN-based PROTACs such as ARV-110 and ARV-471 have drawn a lot of attention for their therapeutic potential in treating cancer and other diseases, the study emphasizes that they have also been thoroughly investigated throughout the world and have been found to be effective in treating a variety of illnesses including viral infections, cardiovascular diseases, immune disorders, and neurodegenerative diseases [45]. Histone deacetylase 6 (HDAC6) may be a therapeutic target for the treatment of a number of disorders, according to a recent study. According to the study, since HDAC6 is essential for the activation of the NLRP3 inflammasome, targeting it may be useful in the treatment of inflammatory illnesses. In order to accomplish this, Cao Z et al. (2021) created an HDAC6 degrader with minimal cytotoxicity using the PROTAC approach, which targets proteolysis. They did this by combining pomalidomide, a CRBN E3 ligand, with a selective HDAC6 inhibitor produced from the natural product indirubin. Multiple cell lines, including active THP-1 cells, had their HDAC6 levels efficiently and arbitrarily decreased by the HDAC6 degrader [46]. Similarly to antibody–drug conjugates (ADCs), antibody–PROTAC conjugates have become a possible method for the selective administration of a broad-spectrum PROTAC to particular cell types. Due to their capacity to deliver cytotoxic drugs to cancer cells only, ADCs have become more and more prominent in the treatment of cancer [47]. He and colleagues created the aptamer–PROTAC conjugate (APC) in their 2021 study by affixing a BET-targeting PROTAC to the nucleic acid aptamer AS1411 (AS) with a cleavable linker. By improving the molecule’s (APR) capacity to target tumors in an MCF-7 xenograft model, this design also reduced toxicity while enhancing BET degradation and anti-tumor effects. The researchers used this method to improve the molecule’s distribution and selectivity, highlighting its potential for use in medicinal applications in the future [48]. Proteolysis-targeting chimeras (PROTACs) have become a promising approach for investigating pharmacological targets that are difficult to target with conventional methods. In recent years, these chimeric molecules have shown effective targeting capabilities, overcoming the challenges posed by traditional approaches. With conventional therapies, medication resistance is frequently unavoidable; nevertheless, this unique technique offers a means of overcoming it. In order to combat acquired drug resistance, PROTACs offer a potentially effective approach by promoting the targeted degradation of particular proteins.References
- Yan, G.; Zhong, X.; Yue, L.; Pu, C.; Shan, H.; Lan, S.; Zhou, M.; Hou, X.; Yang, J.; Li, R. Discovery of a PROTAC targeting ALK with in vivo activity. Eur. J. Med. Chem. 2021, 212, 113150.
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559.
- Flanagan, J.J.; Neklesa, T.K. Targeting nuclear receptors with PROTAC degraders. Mol. Cell. Endocrinol. 2019, 493, 110452.
- Keen, A.C.; Jörg, M.; Halls, M.L. The application of targeted protein degradation technologies to G protein-coupled receptors. Br. J. Pharmacol. 2023.
- Webb, T.; Craigon, C.; Ciulli, A. Targeting epigenetic modulators using PROTAC degraders: Current status and future perspective. Bioorg. Med. Chem. Lett. 2022, 63, 128653.
- Au, Y.Z.; Wang, T.; Sigua, L.H.; Qi, J. Peptide-based PROTAC: The predator of pathological proteins. Cell Chem. Biol. 2020, 27, 637–639.
- Kumar, D.F.; Yaffe, D.; Olshina, M.A.; Ben-Nissan, G.; Sharon, M. The contribution of the 20S proteasome to proteostasis. Biomolecules 2019, 9, 5190.
- Bondeson, D.P.; Crews, C.M. Targeted protein degradation by small molecules. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 107–123.
- Nowak, R.P.; DeAngelo, S.L.; Buckley, D.; He, Z.; Donovan, K.A.; An, J.; Safaee, N.; Jedrychowski, M.P.; Ponthier, C.M.; Ishoey, M. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nat. Chem. Biol. 2018, 14, 706–714.
- Lu, J.; Qian, Y.; Altieri, M.; Dong, H.; Wang, J.; Raina, K.; Hines, J.; Winkler, J.D.; Crew, A.P.; Coleman, K.; et al. Hijacking the E3 ubiquitin ligase cereblon to efficiently target BRD4. Chem. Biol. 2015, 22, 755–763.
- Kaefer, A.; Yang, J.; Noertersheuser, P.; Mensing, S.; Humerickhouse, R.; Awni, W.; Xiong, H. Mechanism-based pharmacokinetic/pharmacodynamic meta-analysis of navitoclax (ABT-263) induced thrombocytopenia. Cancer Chemother. Pharmacol. 2014, 74, 593–602.
- Zhu, H.; Wang, J.; Zhang, Q.; Pan, X.; Zhang, J. Novel strategies and promising opportunities for targeted protein degradation: An innovative therapeutic approach to overcome cancer resistance. Pharmacol. Ther. 2023, 244, 108371.
- Fan, R.; Tao, X.; Zhai, X.; Zhu, Y.; Li, Y.; Chen, Y.; Dong, D.; Yang, S.; Lv, L. Application of aptamer-drug delivery system in the therapy of breast cancer. Biomed. Pharmacoth. 2023, 161, 114444.
- Wu, J.; Wang, W.; Leung, C.H. Computational strategies for PROTAC drug discovery. Acta Mater. Med. 2023, 2, 42–53.
- Zhou, B.; Hu, J.; Xu, F.; Chen, Z.; Bai, L.; Fernandez-Salas, E.; Lin, M.; Liu, L.; Yang, C.-Y.; Zhao, Y. Discovery of a small-molecule degrader of bromodomain and extra-terminal (BET) proteins with picomolar cellular potencies and capable of achieving tumor regression. J. Med. Chem. 2018, 61, 462–481.
- Link, J.O.; Taylor, J.G.; Xu, L.; Mitchell, M.; Guo, H.; Liu, H.; Kato, D.; Kirschberg, T.; Sun, J.; Squires, N. Discovery of ledipasvir (GS-5885): A potent, once-daily oral NS5A inhibitor for the treatment of hepatitis C virus infection. J. Med. Chem. 2014, 57, 2033–2046.
- Maniaci, C.; Hughes, S.J.; Testa, A.; Chen, W.; Lamont, D.J.; Rocha, S.; Alessi, D.R.; Romeo, R.; Ciulli, A. Homo-PROTACs: Bivalent small-molecule dimerizers of the VHL E3 ubiquitin ligase to induce self-degradation. Nat. Commun. 2017, 8, 830.
- Dogheim, G.M.; Amralla, M.T. Proteolysis Targeting Chimera (PROTAC) as a promising novel therapeutic modality for the treatment of triple-negative breast cancer (TNBC). Drug Dev. Res. 2023.
- Li, J.W.; Zheng, G.; Kaye, F.J.; Wu, L. PROTAC therapy as a new targeted therapy for lung cancer. Mol. Ther. 2022, 31, 647–656.
- Lospinoso Severini, L.; Bufalieri, F.; Infante, P.; Di Marcotullio, L. Proteolysis-Targeting Chimera (PROTAC): Is the Technology Looking at the Treatment of Brain Tumors? Front. Cell Devel. Biol. 2022, 10, 291.
- Wang, C.; Zhang, Y.; Deng, J.; Liang, B.; Xing, D. Developments of PROTACs technology in immune-related diseases. Europ. J. Med. Chem. 2023, 249, 115127.
- Shukla, S.K.; Singh, G.; Ahmad, S.; Pant, P. Infections, genetic and environmental factors in pathogenesis of autoimmune thyroid diseases. Microb. Pathog. 2018, 116, 279–288.
- Kargbo, R.B. PROTAC Degradation of IRAK4 for the Treatment of Cancer. ACS. Med. Chem. Lett. 2019, 10, 1370–1371.
- Nunes, J.; McGonagle, G.A.; Eden, J.; Kiritharan, G.; Touzet, M.; Lewell, X.; Emery, J.; Eidam, H.; Harling, J.D.; Anderson, N.A. Targeting IRAK4 for degradation with PROTACs. ACS Med. Chem. Lett. 2019, 10, 1081–1085.
- Humphreys, P.G.; Bamborough, P.; Chung, C.W.; Craggs, P.D.; Gordon, L.; Grandi, P.; Hayhow, T.G.; Hussain, J.; Jones, K.L.; Lindon, M.; et al. Discovery of a potent, cell penetrant, and selective p300/CBP-associated factor (PCAF)/general control nonderepressible 5 (GCN5) bromodomain chemical probe. J. Med. Chem. 2017, 60, 695–709.
- Choi, W.M.; Choi, J.; Lim, Y.S. Hepatitis B: Epidemiology, natural history, and diagnosis. In Comprehensive Guide to Hepatitis Advances; Academic Press: Cambridge, MA, USA, 2023; pp. 183–203.
- Kar, A.; Samanta, A.; Mukherjee, S.; Barik, S.; Biswas, A. The HBV web: An insight into molecular interactomes between the hepatitis B virus and its host en route to hepatocellular carcinoma. J. Med. Virol. 2023, 95, e28436.
- Schollmeier, A.; Glitscher, M.; Hildt, E. Relevance of HBx for Hepatitis B Virus-Associated Pathogenesis. Int. J. Mol. Sci. 2023, 24, 4964.
- Ahmad, H.; Zia, B.; Husain, H.; Husain, A. Recent Advances in PROTAC-Based Antiviral Strategies. Vaccines 2023, 11, 270.
- Yang, N.; Kong, B.; Zhu, Z.; Huang, F.; Zhang, L.; Lu, T.; Chen, Y.; Zhang, Y.; Jiang, Y. Recent advances in targeted protein degraders as potential therapeutic agents. Mol. Divers. 2023, 1–25.
- Yang, J.; Ruan, Y.; Wang, D.; Fan, J.; Luo, N.; Chen, H.; Li, X.; Chen, W.; Wang, X. VHL-recruiting PROTAC attenuates renal fibrosis and preserves renal function via simultaneous degradation of Smad3 and stabilization of HIF-2α. Cell. Biosci. 2022, 12, 203.
- Boeckmans, J.; Gatzios, A.; Schattenberg, J.M.; Koek, G.H.; Rodrigues, R.M.; Vanhaecke, T. PNPLA3 I148M and response to treatment for hepatic steatosis. A systematic review. Liver Int. 2023, 43, 975–988.
- Zebisch, M.; Xu, Y.; Krastev, C.; MacDonald, B.T.; Chen, M.; Gilbert, R.J.; He, X.; Jones, E.Y. Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat. Commun. 2013, 4, 2787.
- Bond, M.J.; Crews, C.M. Proteolysis targeting chimeras (PROTACs) come of age: Entering the third decade of targeted protein degradation. RSC Chem. Biol. 2021, 2, 725–742.
- Edmondson, S.D.; Yang, B.; Fallan, C. Proteolysis targeting chimeras (PROTACs) in ‘beyond rule-of-five’chemical space: Recent progress and future challenges. Bioorg. Med. Chem. Lett. 2019, 29, 1555–1564.
- Vignali, V.; Hines, P.A.; Cruz, A.G.; Ziętek, B.; Herold, R. Health horizons: Future trends and technologies from the European Medicines Agency’s horizon scanning collaborations. Front. Med. 2022, 9, 3588.
- Kargbo, R.B. Treatment of Cancer and Alzheimer’s Disease by PROTAC Degradation of EGFR. ACS Med. Chem. Lett. 2019, 10, 1098–1099.
- Wang, W.; Zhou, Q.; Jiang, T.; Li, S.; Ye, J.; Zheng, J.; Wang, X.; Liu, Y.; Deng, M.; Ke, D.; et al. A novel small-molecule PROTAC selectively promotes tau clearance to improve cognitive functions in Alzheimer-like models. Theranostics 2021, 11, 5279.
- Tonali, N.; Nencetti, S.; Orlandini, E.; Ciccone, L. Application of PROTAC strategy to TTR-Aβ protein-protein interaction for the development of Alzheimer’s disease drugs. Neural Regen. Res. 2021, 8, 1554.
- Abruzzese, M.P.; Bilotta, M.T.; Fionda, C.; Zingoni, A.; Soriani, A.; Vulpis, E.; Borrelli, C.; Zitti, B.; Petrucci, M.T.; Ricciardi, M.R.; et al. Inhibition of bromodomain and extra-terminal (BET) proteins increases NKG2D ligand MICA expression and sensitivity to NK cell-mediated cytotoxicity in multiple myeloma cells: Role of cMYC-IRF4-miR-125b interplay. J. Hematol. Oncol. 2016, 9, 1–9.
- Kargbo, R.B. PROTAC-Mediated Degradation of Janus Kinase as a Therapeutic Strategy for Cancer and Rheumatoid Arthritis. ACS Med. Chem. Lett. 2021, 12, 945–946.
- Kargbo, R.B. PROTAC Degradation of IRAK4 for the Treatment of Neurodegenerative and Cardiovascular Diseases. ACS Med. Chem. Lett. 2019, 10, 1251–1252.
- Kargbo, R.B. PROTAC Compounds Targeting Androgen Receptor for Cancer Therapeutics: Prostate Cancer and Kennedy’s Disease. ACS Med. Chem. Lett. 2020, 11, 1092–1093.
- Ao, M.; Wu, J.; Cao, Y.; He, Y.; Zhang, Y.; Gao, X.; Xue, Y.; Fang, M.; Wu, Z. The synthesis of PROTAC molecule and new target KAT6A identification of CDK9 inhibitor iCDK9. Chi. Chem. Lett. 2023, 34, 107741.
- Wang, C.; Zhang, Y.; Wu, Y.; Xing, D. Developments of CRBN-based PROTACs as potential therapeutic agents. Eur. J. Med. Chem. 2021, 225, 113749.
- Cao, Z.; Gu, Z.; Lin, S.; Chen, D.; Wang, J.; Zhao, Y.; Li, Y.; Liu, T.; Li, Y.; Wang, Y.; et al. Attenuation of NLRP3 inflammasome activation by indirubin-derived PROTAC targeting HDAC6. ACS Chem. Biol. 2021, 16, 2746–2751.
- Lambert, J.M.; Berkenblit, A. Antibody–drug conjugates for cancer treatment. Annu. Rev. Med. 2018, 69, 191–207.
- He, S.; Gao, F.; Ma, J.; Ma, H.; Dong, G.; Sheng, C. Aptamer-protac conjugates (apcs) for tumor-specific targeting in breast cancer. Angew. Chem. 2021, 133, 23487–23493.
