Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by M. Pilar Gómez-Serranillos.
Ginseng, a medicinal plant of the genus Panax, boasts a rich historical record of usage that dates back to the Paleolithic period. This botanical is extensively acknowledged and consumed in Eastern countries for its therapeutic properties, and, in Western countries, it is becoming increasingly popular as a remedy for fatigue and asthenia.
- ginsenosides
- ginseng
- immunomodulation
1. Introduction
Ginseng, a highly valued herbal medicine, has been used for over 5000 years, predominantly in Far East countries. Emperor Shen-Nung of China was the first to identify ginseng, along with several other herbs, as having medicinal properties, and developed what is considered the first pharmacopoeia in history [1]. Ginseng is the common name for the root of various species of the genus Panax (P.), belonging to the Araliacea family. The genus name, Panax, is derived from the ancient Greek “Panákeia” (Πανάκεια), which translates to “all healing” or “cure of all diseases.” Similarly, the term “ginseng” originates from the Chinese “jen-shen”, which means “plant-man”, possibly referencing the human form of the root [2,3][2][3]. The Greek term “panacea”, which shares the same etymological roots as “panax”, also implies that the ginseng root is a genuine and authentic panacea.
Archaeological research indicates that the ginseng root has been used as a medicinal plant since the Paleolithic period, dating back approximately 60,000 years ago [4]. The use of ginseng as a medicinal plant has been predominantly concentrated in China and other Asian countries, where it grows naturally. In East Asian countries, ginseng has been used as an adaptogenic drug since its discovery in the Manchurian mountains, more than 5000 years ago [5]. The Shen-Nung Benchau Jing treatise is considered the oldest pharmacopoeia in the world, and the use of ginseng is already mentioned in it, revealing that its pharmacological potential was already known [6].
2. Phytochemical Characteristic
The genus Panax consists of eleven species, including P. ginseng, P. notoginseng, P. quinquefolius, P. pseudoginseng, P. trifolius, P. zingiberensis, P. stipuleanatus, P. japonicus, P. japonicus var. angustifolius, P. japonicus var. major, and P. japonicus var. bipinnatifidus [14][7]. Morphologically, they are characterized by herbaceous perennial plants with palmate leaves with serrated margins. Generally, ginseng species reach 60–80 cm in height, and produce flowers ranging from white to purple, these flowers appear in an umbel at the apex. In addition, these species have a complex root system, comprising of a short rhizome and many tuberous roots [15][8]. While the most used species for their pharmacological properties are P. ginseng, P. notoginseng, and P. quinquefolius, all these species contain bioactive compounds of pharmacological interest [16][9]. Ginsenosides, the primary bioactive compounds found in ginseng, can be classified into five groups, based on their distinct chemical structures: protopanaxadiol, protopanaxatriol, ocotillo, oleanolic acid, and C-17 side-chain type [17][10]. The structural diversity of ginsenosides is attributed to the presence of various radicals and sugar moieties. Furthermore, the primary structure of some ginsenosides can be modified to produce secondary ginsenosides through natural microbiota [18][11], or by various processes during the preparation of the phytotherapeutic product, such as heating or drying. Some of these molecules can be seen in Figure 21. Additionally, the composition of each ginseng preparation can be influenced by factors such as growth temperature, altitude, and weather conditions, in the different places of cultivation [19][12]. Although ginsenosides are the most extensively studied molecules among the components of ginseng, other bioactive compounds, such as polysaccharides, glycolipoproteins, and alkaloids, also exhibit important pharmacological activities.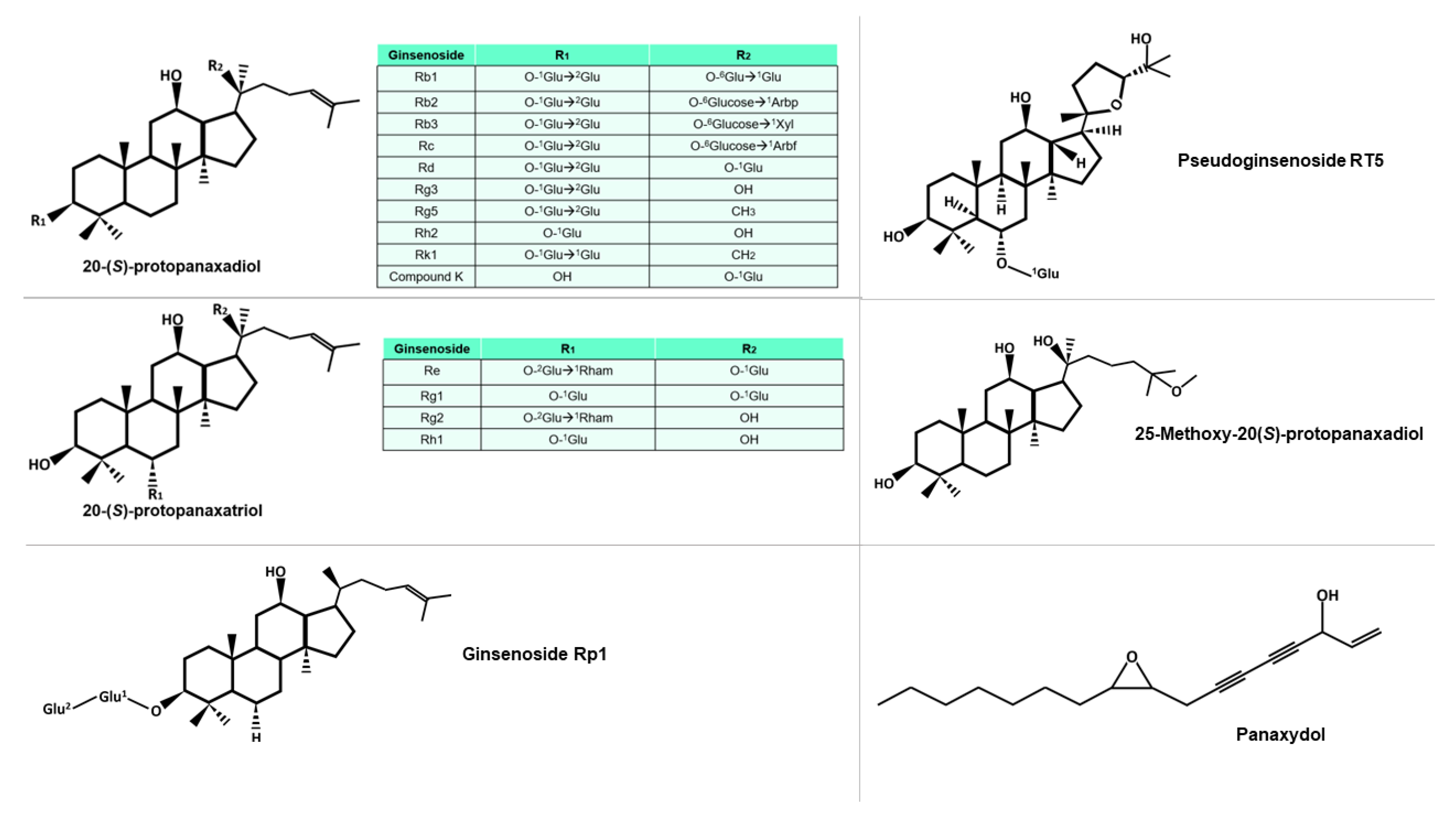
Figure 21. Diversity of protopanaxadiol and protopanaxtriol-type ginsenosides, characterized by the glucidic substituents they possess, including Glu (glucose), Arbp (arabinose in pyranose form), Xyl (xylose), Arbf (arabinose in furanose form), and Rham (rhamnose). The superscript notation of the sugar indicates the specific carbon involved in the bond. Other molecules mentioned in this study are also included in the figure.
3. Immunomodulatory Activity
The immune system plays a vital role in defending the body against external threats. In humans, it is comprised of two distinct components, namely, the innate immune system and the adaptive immune system. Each system is equipped with unique cells and molecules to perform its specific functions. Macrophages and Natural Killer Cells (NK cells) are well-known cells of the innate immune system, whereas T lymphocytes and B lymphocytes are good examples of the adaptive immune system. To achieve successful immunity against pathogens, effective communication between these two systems is essential. In this context, the present discussion focuses on how various molecules found in ginseng can influence the immune system, and a summary is provided in Table 1. Ginseng has been extensively studied for its bioactivity in modulating the immune system, a property also observed in other medicinal plants [23][16]. Initially, it was suggested that ginseng polysaccharides were responsible for this immunomodulatory effect [9][17]. However, subsequent investigations have revealed the involvement of various ginsenosides, including RT5, Rh2, oleanolic acid β-D-glucopyranosyl ester [24][18], Rh1 [25][19], Rg3 [26][20], and Rb1 [27][21], in triggering this biological response. P. ginseng extracts have demonstrated noteworthy pharmacological bioactivities, including the improvement of macrophages’ phagocytic activity and enhanced production of NO [28,29][22][23]. Furthermore, ginseng extracts have been shown to boost interleukin 12 (IL-12) release [30][24]. Scaglione et al. [31][25] conducted a study at two levels, in vivo and with humans, demonstrating interesting results that can be immediately applied in clinical treatments, such as improvements in chemotaxis, phagocytosis index, and phagocytosis fraction. Additionally, different ginseng extracts have been observed to regulate various cytokines and molecules involved in immunomodulation, including IL-1α, IL-1β, IL-6, tumor necrosis factor α (TNF-α), NO, inducible nitric oxide synthase (iNOS), or cyclooxygenase-2 (COX-2) [32,33][26][27]. Therefore, ginseng extracts possess bioactive compounds that can regulate the immune system, resulting in a normalization of its functioning. The observed effect may be attributed to the presence of specific compounds or a synergistic activity among them, which may produce a more potent effect. The earliest investigations into the immunomodulatory effects of ginseng attributed its bioactivity to its polysaccharide content [34,35,36,37,38,39][28][29][30][31][32][33]. The underlying mechanisms of action have been partially elucidated, with TNF-α being the primary stimulus, and other molecules, such as NO, IL-6, and IL-1β, also being stimulated [40][34]. In addition to the increased production of these immune-associated molecules, ginseng extracts have been shown to induce a Th1 immune response and activate pathways, such as nuclear factor κB (NF-κB), mitogen-activated protein kinases (MAPK), and phosphatidylinositol 3-kinase (PI3K) [14][7]. The primary distinction between ginseng extract responses depends on the mode of extraction and presentation, as well as the molecular weight of bioactive compounds. Notably, a conflicting effect was observed in vivo by Azike et al., who reported that, while the aqueous extract of ginseng polysaccharides increased TNF-α and NO levels, it also inhibited the physiological rise of these proinflammatory mediators induced by lipopolysaccharide (LPS). TNF-α levels were determined by Enzyme-Linked InmunoSorbent Assay (ELISA), and NO production was estimated by assessing nitrite accumulation with the Griess reagent. Treated animals displayed a reduction of around 50% in NO production, compared to non-treated animals [37][31]. This effect can be explained by considering that ginseng exerts an immunomodulatory action, instead of a stimulatory or inhibitory effect. Ginsan, an acidic polysaccharide, has been found to possess unique abilities in stimulating the production of inflammatory mediators, such as NO, by upregulating iNOS, which sets it apart from other ginseng extracts [41][35]. However, ginsan also exhibits other bioactivities related to the immune response, such as the induction of T helper type 1 (Th1) cells and the release of cytokines produced by macrophages [42][36]. In addition, ginsan has multiple immunomodulatory effects on dendritic cells, including enhancing the expression of cluster of differentiation 86 (CD86) on their surface and increasing the levels of IL-12 and TNF-α secreted by them [43][37]. Recent studies have challenged the previous notion that polysaccharides are solely responsible for the immunomodulatory bioactivity of ginseng. Instead, ginsenosides and ginsenoside-like molecules have been shown to play an important role through distinct, and sometimes overlapping, mechanisms [44,45][38][39]. In vitro studies have demonstrated that ginsenoside RT5 and ginsenoside Rh2 increase IL-2 production. Interestingly, oleanane-type triterpenoids have been found to exhibit stronger immunomodulatory effects than those of the dammarane type [24][18]. Additionally, ginsenoside Rh2 has been found to increase the number of T cells in mice with melanoma, linking its immunomodulatory effect with anti-cancer properties [46][40]. Animal studies involving ginsenoside Rb2 have also revealed a higher survival rate and reduced tumor size [46][40]. In vitro studies have demonstrated the immunomodulatory activity of ginsenoside Rh1. Its action is complementary to that of polysaccharides, which regulate proinflammatory mediators, such as TNF-α or IL-6. Ginsenoside Rh1 reduces the expression of various proinflammatory mediators, including TNF-α, IL-1β, IL-6, IL-17, and NO. Additionally, it suppresses enzymes such as matrix metalloproteinase 1 (MMP-1), iNOS, and COX-2 [47,48][41][42]. In obese mice, Rh1 has been shown to exhibit immunomodulatory properties, by suppressing proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 [49][43]. In hairless female mice, oral administration of Rh1 resulted in reduced IL-6 and immunoglobulin E (IgE) levels in peripheral blood analysis [50][44]. These findings suggest that Rh1 has some level of bioavailability through this route of administration; however, further research is necessary to clarify this point. In various studies, other ginsenosides isolated as pure compounds have also demonstrated immunomodulatory properties. For instance, Rg3 has been found to enhance Fc gamma receptor-mediated phagocytosis in macrophages through mechanisms related to the activation of extracellular signal-regulated kinase 1/2 (ERK 1/2) and p38 [25][19]. Additionally, Rg3 has been shown to regulate cytokines and transcription factors, thereby maintaining homeostasis [51][45]. Ginsenoside Rg3 has been shown, in a study with patients with non-small cell lung cancer, to improve the immune response against COVID-19 by modulating the immune system against this disease. The same study shows the ability of ginsenoside Rg3 to regulate cell cycle, suggesting a possible application against different types of cancer [52][46]. Another compound, Rb1, has been found to increase both humoral and cell-mediated immune responses through the induction of antigen-presenting cells, which secrete TNF-α, and T cells, which secrete interferon gamma (IFN-γ) and IL-10. Rb1 also induces the production of immunoglobulins such as IgA, IgG1, and IgG2, and potentiates virus-triggered IFN-γ expression [27][21]. In another study, Rb1 was found to ameliorate the expression of TNF-α and IL-6 [53][47]. Rg1 has demonstrated many mechanisms through which it can carry out its activity related to immunomodulation. Studies, both in vivo and in vitro, have shown that Rg1 can activate the nuclear factor E2-related factor 2 (Nrf2) signaling pathway, which generates protection of the liver from toxins and disease [54,55,56][48][49][50]. Furthermore, both ginsenoside Rg1 and ginseng extracts have been shown to improve NK cells activity [57][51]. One of the main problems in therapy with ginsenosides is the great difficulty to absorb them. In fact, the bioavailability of ginsenosides from the intestinal mucosa is very low, and its transport through intestinal mucosa to blood is energy-dependent and non-saturable [3]. Compound K, which is not a main ginsenoside, but is closely related to them and derived from their biotransformation, is of clinical significance, due to its better bioavailability and numerous immune system activities. It attenuates NF-κB by modulating the protein kinase B (PKB or Akt)-mediated inflammatory gene expression [58][52], and regulates cytokines and other immune molecules such as IL-1β, IL-6, TNF-α, COX-2, and iNOS [59,60,61][53][54][55]. However, Compound K has also been shown to suppress humoral immune response of Th1 cells and suppresses the expression of matrix metalloproteinases and receptor activator of NF-κB ligand (RANKL) [62][56]. Additionally, it can inhibit β-arrestin2, hindering the transformation of macrophages from type M1 to type M2 [63][57].Table 1.
Immunomodulatory bioactivity of ginseng extracts and compounds. An increase is represented by (↑), and a decrease is represented by (↓).
| Species | Molecular Group | Compound/Extract | Experimental Model | Result | Ref. |
|---|---|---|---|---|---|
| Panax quinquefolius L. | Polysaccharides | Extract | Wistar rats | ↑ Macrophages activity | [34][28] |
| Polysaccharides | Extract | Human peripheral blood mononuclear cells | ↑ Pro-inflammatory cytokines | [35][29] | |
| Polysaccharides | Extract | Mouse 3T3-L1 preadipocytes | Cytokines regulation | [36][30] | |
| Polysaccharides | Extract | Sprague–Dawley rats Murine RAW 264.7 macrophage cell line |
Cytokines regulation | [37][31] | |
| Panax ginseng C.A. Meyer | Polysaccharides | Acidic fraction | C57BL/6 mice macrophages | Cytokines regulation | [38][32] |
| Polysaccharides | Acidic fraction | C57BL/6 mice | Enhanced phagocytic effect | [41][35] | |
| Polysaccharides | Acidic fraction | C57BL/6 mice dendritic cells | ↑ CD86 | [43][37] | |
| Ginsenosides | Rh1 | Murine RAW 264.7 macrophage cell line |
Glucocorticoid receptor stimulus | [47][41] | |
| Ginsenosides | Rh1 | Hartley guinea pigs, SD rats, and ICR mice | ↓ NO ↓ PGE2 | [48][42] | |
| Ginsenosides | Rh1 | Mouse embryo fibroblasts 3T3-L1 cells | ↓ TNF-α ↓ IL-1β ↓ IL-6 | [49][43] | |
| Ginsenosides | Rh1 | Hairless mice | ↓ Infiltration of inflammatory cells ↓ IgE levels |
[50][44] | |
| Ginsenosides | Rb1 | EV71 mice model | ↑ Cellular immune response ↑ Humoral immune response |
[27][21] | |
| Ginsenosides | Rg1 | C57BL/6 mice C57BL/6 mice hepatocytes |
↑ Nrf2 ↑ Detoxifying enzymes |
[55][49] | |
| Ginsenosides | Rg3 | BALB/c mice | Improve immune system | [51][45] | |
| Ginsenosides | Rg3 | Patients with non-small cell lung cancer | Regulate cell cycle | [52][46] | |
| Ginsenosides | Standardized G-115 extract |
BALB/c pathogen-free mice | ↑TLR4 | [40][34] | |
| Ginsenosides | Compound K | Murine RAW 264.7 macrophage cell line Human Embryonic Kidney cell line (HEK293 cells) |
↓ iNOS ↓ TNF-α | [58][52] | |
| Ginsenosides | Compound K | Sprague–Dawley rats Kunming mice |
Cytokines regulation | [61][55] | |
| Ginsenosides | Compound K | DBA/1 OlaHsd mice | ↓ Th1 response (in arthritis) |
[62][56] | |
| Ginsenosides | Compound K | DBA/1 mice | Alleviates inflammatory response | [63][57] | |
| - | Extract | Clinical trial | ↑ Chemotaxis | [31][25] | |
| - | Extract | Murine RAW 264.7 macrophage cell line BALB/c mice |
Cytokines regulation | [28][22] | |
| - | Extract | Balb/C mice C57 B1/6J mice C57 B1/6J nu/nu mice |
↑ Antibody formation ↑ NK |
[29][23] | |
| - | Extract | Murine RAW 264.7 macrophage cell line |
Cytokines regulation | [32][26] | |
| - | Extract | Murine RAW 264.7 macrophage cell line |
↑ Immunomodulators | [33][27] | |
| Panax ginseng C.A. Meyer Eleutherococcus senticosus Rupr. & Maxim |
- | Extract | Mouse J774A.1 macrophages | ↑ lL-12 | [30][24] |
References
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 2017, 107, 362–372.
- Attele, A.S.; Wu, J.A.; Yuan, C.-S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693.
- Leung, K.W.; Wong, A.S.-T. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20.
- González-Burgos, E.; Fernandez-Moriano, C.; Gómez-Serranillos, M.P. Potential Neuroprotective Activity of Ginseng in Parkinson’s Disease: A Review. J. Neuroimmune Pharmacol. 2014, 10, 14–29.
- Zhang, H.; Abid, S.; Ahn, J.C.; Mathiyalagan, R.; Kim, Y.-J.; Yang, D.-C.; Wang, Y. Characteristics of Panax ginseng Cultivars in Korea and China. Molecules 2020, 25, 2635.
- Ratan, Z.A.; Youn, S.H.; Kwak, Y.-S.; Han, C.-K.; Haidere, M.F.; Kim, J.K.; Min, H.; Jung, Y.-J.; Hosseinzadeh, H.; Hyun, S.H.; et al. Adaptogenic effects of Panax ginseng on modulation of immune functions. J. Ginseng Res. 2021, 45, 32–40.
- Ghosh, R.; Bryant, D.L.; Farone, A.L. Panax quinquefolius (North American Ginseng) Polysaccharides as Immunomodulators: Current Research Status and Future Directions. Molecules 2020, 25, 5854.
- Yang, Y.; Ju, Z.; Yang, Y.; Zhang, Y.; Yang, L.; Wang, Z. Phytochemical analysis of Panax species: A review. J. Ginseng Res. 2021, 45, 1–21.
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2015; Volume 1, pp. 191–193.
- Yang, W.-Z.; Hu, Y.; Wu, W.-Y.; Ye, M.; Guo, D.-A. Saponins in the genus Panax L. (Araliaceae): A systematic review of their chemical diversity. Phytochemistry 2014, 106, 7–24.
- Chen, Z.; Zhang, Z.; Liu, J.; Qi, H.; Li, J.; Chen, J.; Huang, Q.; Liu, Q.; Mi, J.; Li, X. Gut Microbiota: Therapeutic Targets of Ginseng Against Multiple Disorders and Ginsenoside Transformation. Front. Cell. Infect. Microbiol. 2022, 12, 456.
- Dong, T.T.X.; Cui, X.M.; Song, Z.H.; Zhao, K.J.; Ji, Z.N.; Lo, C.K.; Tsim, K.W.K. Chemical Assessment of Roots of Panax notoginseng in China: Regional and Seasonal Variations in Its Active Constituents. J. Agric. Food Chem. 2003, 51, 4617–4623.
- Yang, W.-Z.; Ye, M.; Qiao, X.; Liu, C.-F.; Miao, W.-J.; Bo, T.; Tao, H.-Y.; Guo, D.-A. A strategy for efficient discovery of new natural compounds by integrating orthogonal column chromatography and liquid chromatography/mass spectrometry analysis: Its application in Panax ginseng, Panax quinquefolium and Panax notoginseng to characterize 437 potential new ginsenosides. Anal. Chim. Acta 2012, 739, 56–66.
- Liu, J.; Wu, Y.; Ma, W.; Zhang, H.; Meng, X.; Zhang, H.; Guo, M.; Ling, X.; Li, L. Anti-Inflammatory Activity of Panax notoginseng Flower Saponins Quantified Using LC/MS/MS. Molecules 2023, 28, 2416.
- Yao, C.-L.; Pan, H.-Q.; Wang, H.; Yao, S.; Yang, W.-Z.; Hou, J.-J.; Jin, Q.-H.; Wu, W.-Y.; Guo, D.-A. Global profiling combined with predicted metabolites screening for discovery of natural compounds: Characterization of ginsenosides in the leaves of Panax notoginseng as a case study. J. Chromatogr. A 2018, 1538, 34–44.
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396.
- Karra, A.G.; Konstantinou, M.; Tzortziou, M.; Tsialtas, I.; Kalousi, F.D.; Garagounis, C.; Hayes, J.M.; Psarra, A.-M.G. Potential Dissociative Glucocorticoid Receptor Activity for Protopanaxadiol and Protopanaxatriol. Int. J. Mol. Sci. 2018, 20, 94.
- Vinh, L.B.; Park, J.U.; Duy, L.X.; Nguyet, N.T.M.; Yang, S.Y.; Kim, Y.R.; Kim, Y.H. Ginsenosides from Korean red ginseng modulate T cell function via the regulation of NF-AT-mediated IL-2 production. Food Sci. Biotechnol. 2018, 28, 237–242.
- Tam, D.N.H.; Truong, D.H.; Nguyen, T.T.H.; Quynh, L.N.; Tran, L.; Nguyen, H.D.; Shamandy, B.E.; Le, T.M.H.; Tran, D.K.; Sayed, D.; et al. Ginsenoside Rh1: A Systematic Review of Its Pharmacological Properties. Planta Medica 2018, 84, 139–152.
- Xin, C.; Kim, J.; Quan, H.; Yin, M.; Jeong, S.; Choi, J.I.; Jang, E.A.; Lee, C.H.; Kim, D.H.; Bae, H.B. Ginsenoside Rg3 promotes Fc gamma receptor-mediated phagocytosis of T bacteria by macrophages via an extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent mechanism. Int. Immunopharmacol. 2019, 77, 105945.
- Kang, N.; Gao, H.; He, L.; Liu, Y.; Fan, H.; Xu, Q.; Yang, S. Ginsenoside Rb1 is an immune-stimulatory agent with antiviral activity against enterovirus 71. J. Ethnopharmacol. 2021, 266, 113401.
- Lim, T.-G.; Jang, M.; Cho, C.-W.; Hong, H.-D.; Kim, K.-T.; Lee, S.-Y.; Jung, S.K.; Rhee, Y.K. White ginseng extract induces immunomodulatory effects via the MKK4-JNK pathway. Food Sci. Biotechnol. 2016, 25, 1737–1744.
- Jie, Y.H.; Cammisuli, S.; Baggiolini, M. Immunomodulatory effects of Panax Ginseng C.A. Meyer in the mouse. Agents Actions 1984, 15, 386–391.
- Wang, H.; Actor, J.K.; Indrigo, J.; Olsen, M.; Dasgupta, A. Asian and Siberian ginseng as a potential modulator of immune function: An in vitro cytokine study using mouse macrophages. Clin. Chim. Acta 2003, 327, 123–128.
- Scaglione, F.; Ferrara, F.; Dugnani, S.; Falchi, M.; Santoro, G.; Fraschini, F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Exp. Clin. Res. 1990, 16, 537–542.
- Jang, H.I.; Shin, H.M. Wild Panax Ginseng (Panax ginseng C.A. Meyer) Protects Against Methotrexate–Induced Cell Regression by Enhancing the Immune Response in RAW 264.7 Macrophages. Am. J. Chin. Med. 2010, 38, 949–960.
- Um, Y.; Eo, H.J.; Kim, H.J.; Kim, K.; Jeon, K.S.; Jeong, J.B. Wild simulated ginseng activates mouse macrophage, RAW264.7 cells through TRL2/4-dependent activation of MAPK, NF kappaB and PI3K/AKT pathways. J. Ethnopharmacol. 2020, 263, 113218.
- Assinewe, V.; Arnason, J.; Aubry, A.; Mullin, J.; Lemaire, I. Extractable polysaccharides of Panax quinquefolius L. (North American ginseng) root stimulate TNFa production by alveolar macrophages. Phytomedicine 2002, 9, 398–404.
- Lemmon, H.R.; Sham, J.; Chau, L.A.; Madrenas, J. High molecular weight polysaccharides are key immunomodulators in North American ginseng extracts: Characterization of the ginseng genetic signature in primary human immune cells. J. Ethnopharmacol. 2012, 142, 1–13.
- Wilson, S.A.F.; Wong, M.H.T.; Stryjecki, C.; De Boer, A.; Lui, E.M.K.; Mutch, D. Unraveling the adipocyte inflammomodulatory pathways activated by North American ginseng. Int. J. Obes. 2013, 37, 350–356.
- Azike, C.G.; Charpentier, P.A.; Lui, E.M. Stimulation and Suppression of Innate Immune Function by American Ginseng Polysaccharides: Biological Relevance and Identification of Bioactives. Pharm. Res. 2015, 32, 876–897.
- Shin, J.Y.; Song, J.Y.; Yun, Y.S.; Yang, H.O.; Rhee, D.K.; Pyo, S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol. Immunotoxicol. 2002, 24, 469–482.
- Pannacci, M.; Lucini, V.; Colleoni, F.; Martucci, C.; Grosso, S.; Sacerdote, P.; Scaglione, F. Panax ginseng C.A. Mayer G115 modulates pro-inflammatory cytokine production in mice throughout the increase of macrophage toll-like receptor 4 expression during physical stress. Brain, Behav. Immun. 2006, 20, 546–551.
- Lim, D.; Bae, K.; Jung, I.; Kim, C.; Yun, Y.; Song, J. Anti-Septicaemic Effect of Polysaccharide from Panax ginseng by Macrophage Activation. J. Infect. 2002, 45, 32–38.
- Choi, H.S.; Kim, K.H.; Sohn, E.; Park, J.D.; Kim, B.O.; Moon, E.Y.; Rhee, D.K.; Pyo, S. Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-kappaB pathway. Biosci. Biotechnol. Biochem. 2008, 72, 1817–1825.
- Kim, K.-H.; Lee, Y.-S.; Jung, I.-S.; Park, S.-Y.; Chung, H.-Y.; Lee, I.-R.; Yun, Y.-S. Acidic Polysaccharide from Panax ginseng, Ginsan, Induces Th1 Cell and Macrophage Cytokines and Generates LAK Cells in Synergy with rIL-2. Planta Medica 1998, 64, 110–115.
- Kim, M.-H.; Byon, Y.-Y.; Ko, E.-J.; Song, J.-Y.; Yun, Y.-S.; Shin, T.; Joo, H.-G. Immunomodulatory Activity of Ginsan, a Polysaccharide of Panax Ginseng, on Dendritic Cells. Korean J. Physiol. Pharmacol. 2009, 13, 169–173.
- You, L.; Cha, S.; Kim, M.-Y.; Cho, J.Y. Ginsenosides are active ingredients in Panax ginseng with immunomodulatory properties from cellular to organismal levels. J. Ginseng Res. 2022, 46, 711–721.
- Luo, H.; Chen, J.; Su, C.; Zha, L. Advances in the Bioactivities of Phytochemical Saponins in the Prevention and Treatment of Atherosclerosis. Nutrients 2022, 14, 4998.
- Wang, M.; Yan, S.-J.; Zhang, H.-T.; Li, N.; Liu, T.; Zhang, Y.-L.; Li, X.-X.; Ma, Q.; Qiu, X.-C.; Fan, Q.-Y.; et al. Ginsenoside Rh2 enhances the antitumor immunological response of a melanoma mice model. Oncol. Lett. 2016, 13, 681–685.
- Li, J.; Du, J.; Liu, D.; Cheng, B.; Fang, F.; Weng, L.; Wang, C.; Ling, C. Ginsenoside Rh1 potentiates dexamethasone’s anti-inflammatory effects for chronic inflammatory disease by reversing dexamethasone-induced resistance. Arthritis Res. Ther. 2014, 16, R106.
- Park, E.-K.; Choo, M.-K.; Han, M.J.; Kim, D.-H. Ginsenoside Rh1 Possesses Antiallergic and Anti-Inflammatory Activities. Int. Arch. Allergy Immunol. 2004, 133, 113–120.
- Gu, W.; Kim, K.-A.; Kim, D.-H. Ginsenoside Rh1 Ameliorates High Fat Diet-Induced Obesity in Mice by Inhibiting Adipocyte Differentiation. Biol. Pharm. Bull. 2013, 36, 102–107.
- Zheng, H.; Jeong, Y.; Song, J.; Ji, G.E. Oral administration of ginsenoside Rh1 inhibits the development of atopic dermatitis-like skin lesions induced by oxazolone in hairless mice. Int. Immunopharmacol. 2011, 11, 511–518.
- Liu, X.; Zhang, Z.; Liu, J.; Wang, Y.; Zhou, Q.; Wang, S.; Wang, X. Ginsenoside Rg3 improves cyclophosphamide-induced immunocompetence in Balb/c mice. Int. Immunopharmacol. 2019, 72, 98–111.
- Zhuang, Z.; Chen, Q.; Zhong, X.; Chen, H.; Yu, R.; Tang, Y. Ginsenoside Rg3, a promising agent for NSCLC patients in the pandemic: A large-scale data mining and systemic biological analysis. J. Ginseng Res. 2023, 47, 291–301.
- Lu, S.; Zhang, Y.; Li, H.; Zhang, J.; Ci, Y.; Han, M. Ginsenoside Rb1 can ameliorate the key in nti-in cytokines TNF-alpha and IL-6 in a cancer cachexia mouse model. BMC Complement. Med. Ther. 2020, 20, 11.
- Shi, J.; Weng, J.-H.; Mitchison, T.J. Immunomodulatory drug discovery from herbal medicines: Insights from organ-specific activity and xenobiotic defenses. Elife 2021, 10, e73673.
- Ning, C.; Gao, X.; Wang, C.; Kong, Y.; Liu, Z.; Sun, H.; Sun, P.; Huo, X.; Ma, X.; Meng, Q.; et al. Ginsenoside Rg1 protects against acetaminophen-induced liver injury via activating Nrf2 signaling pathway in vivo and in vitro. Regul. Toxicol. Pharmacol. 2018, 98, 58–68.
- Gao, Y.; Chu, S.; Zhang, Z.; Chen, N. Hepataprotective effects of ginsenoside Rg1—A review. J. Ethnopharmacol. 2017, 206, 178–183.
- Kang, S.W.; Min, H. Ginseng, the ‘Immunity Boost’: The Effects of Panax ginseng on Immune System. J. Ginseng Res. 2012, 36, 354–368.
- Lee, J.O.; Choi, E.; Shin, K.K.; Hong, Y.H.; Kim, H.G.; Jeong, D.; Hossain, M.A.; Kim, H.S.; Yi, Y.S.; Kim, D.; et al. Compound K, a ginsenoside metabolite, plays an antiinflammatory role in macrophages by targeting the AKT1-mediated signaling pathway. J. Ginseng Res. 2019, 43, 154–160.
- Sharma, A.; Lee, H.-J. Ginsenoside Compound K: Insights into Recent Studies on Pharmacokinetics and Health-Promoting Activities. Biomolecules 2020, 10, 1028.
- Yang, X.-D.; Yang, Y.-Y.; Ouyang, D.-S.; Yang, G.-P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia 2015, 100, 208–220.
- Chen, J.; Si, M.; Wang, Y.; Liu, L.; Zhang, Y.; Zhou, A.; Wei, W. Ginsenoside metabolite compound K exerts anti-inflammatory and analgesic effects via downregulating COX2. Inflammopharmacology 2018, 27, 157–166.
- Lee, Y.J.; Song, K.Y.; Lee, E.Y.; Kang, H.S.; Song, Y.W. Compound K, a Metabolite of Ginsenosides, Attenuates Collagen-induced Arthritis in Mice. J. Rheum. Dis. 2015, 22, 154–166.
- Wang, R.; Zhang, M.; Hu, S.; Liu, K.; Tai, Y.; Tao, J.; Zhou, W.; Zhao, Z.; Wang, Q.; Wei, W. Ginsenoside metabolite compound-K regulates macrophage function through inhibition of β-arrestin2. Biomed. Pharmacother. 2019, 115, 108909.
More
